Background and overview[1]
3-Morpholinylbenzaldehyde can be used as a pharmaceutical synthesis intermediate. If 3-morpholinobenzaldehyde is inhaled, move the patient to fresh air; if skin contact occurs, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if contact with eyes , you should separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
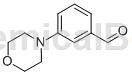
Preparation[1]
The preparation of 3-morpholinobenzaldehyde is as follows:
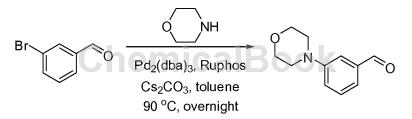
Add 3-bromobenzaldehyde (6.44g, 35.0mmol, 1.00 equivalent), morpholine (9.13g, 105mmol, 3.00 equivalent), tris(dibenzylideneacetone) dipalladium (1.81) into a 500mL round bottom flask. g, 1.75 mmol, 0.05 equiv), dicyclohexyl (2′,6′-diisopropoxybiphenyl-2-yl)phosphine (3.26 g, 7.00 mmol, 0.20 equiv), cesium carbonate (34.2 g , 105 mmol) under nitrogen, 3.00 equiv), toluene (100 mL). The resulting solution was stirred at 90 °C overnight and quenched with water (50 mL). The resulting solution was extracted with dichloromethane (3 × 200 mL), the organic layers were combined, washed with brine (3 × 100 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was chromatographed on a silica gel column using ethyl acetate/petroleum ether (3/17) to obtain 3-morpholinobenzaldehyde (2.10 g, 32% yield) as a yellow oil.
Apply[1]
3-Morpholinylbenzaldehyde can be used as a pharmaceutical synthesis intermediate. For example, prepare the following compounds:
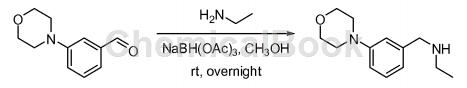
Add 3-morpholinobenzaldehyde (4.58g, 23.9mmol, 1.00 equivalents), ethylamine (5.39g, 120mmol, 5.00 equivalents), and methanol (100mL) into a 250mL round-bottomed flask. The resulting solution was stirred at room temperature for 2 hours. Add triacetoxy�Sodium hydride (15.2g, 72.0mmol, 3.00 equivalents). The resulting solution was stirred at room temperature overnight and quenched with water (50 mL). The resulting solution was extracted with dichloromethane (3 × 100 mL), the organic layers were combined, washed with brine (3 × 100 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was chromatographed on a silica gel column using ethyl acetate/petroleum ether (5/1) to obtain N-(3-morpholinobenzyl)ethylamine (4.00g, 75% yield) as a yellow solid .
Main reference materials
[1] WO2015179559 PYRAZOLE COMPOUNDS AND METHODS OF MAKING AND USING SAME

 微信扫一扫打赏
微信扫一扫打赏

