Background and overview[1]
Methyl 2-bromo-4,5-difluorobenzoate can be used as a pharmaceutical synthesis intermediate. If 2-bromo-4,5-difluorobenzoic acid methyl ester is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse the skin thoroughly with soap and water. If discomfort occurs , seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
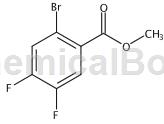
Preparation[1]
Methyl 2-bromo-4,5-difluorobenzoate was prepared as follows: 2-bromo-4,5-difluorobenzoic acid (1 g, 4.22 mmol) was dissolved in dichloromethane (10 mL) and MeOH ( Add trimethylsilyl)diazomethane (2.110 mL, 4.22 mmol) to the mixture in 1 mL) and stir the reaction. 1 hour at room temperature. It was then concentrated to obtain methyl 2-bromo-4,5-difluorobenzoate (1 g, 3.98 mmol, 94% yield) as an oil.
Apply[2]
Methyl 2-bromo-4,5-difluorobenzoate can be used as a pharmaceutical synthesis intermediate. For example, prepare the following compounds:
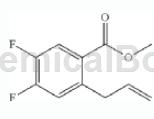
The specific steps are: 2-bromo-4,5-difluorobenzoic acid methyl ester (0.5g, 1.992mmol), allyltributylstannane (0.679mL, 2.191mmol), lithium chloride (0.169g, To a mixture of 3.98 mmol) in DMF (1 mL), acetonitrile (10 mL) was added bis(triphenylphosphine)palladium(ii) dichloride (0.070 g, 0.100 mmol). It was then degassed for 2 minutes and then filled with N2. After heating at 90°C for 16 hours, it was quenched with NH4Cl and extracted with EtOAc. The organic layer was washed 5 times with KF solution, then dried over MgSO4, filtered and concentrated to give an oil, which was then purified by biotage, eluting with 10% EtOAc/hexanes to isolate methyl 2-allyl-4,5 -Difluorobenzoate (330 mg, 1.555 mmol, 78% yield) as oil.
Main reference materials
[1] WO2015126737 PYRAZOLOPYRIMIDINEMACROCYCLESASINHIBITORSOFHUMANIMMUNODEFICIENCYVIRUSREPLICATION
[2] US20150232481 Pyrazolopyrimidine macrocycles as inhibitors of human immunodeficiency virus replication

 微信扫一扫打赏
微信扫一扫打赏

