Background and overview[1]
Methyl 3-phenylpropionate is also called methyl phenylpropionate and hydrogenated methyl cinnamate. Ester compounds and their derivatives have important uses in medicine, chemical industry, fuel, etc., such as synthetic perfumes, flavoring agents, detergents, surfactants, etc.
Preparation[1-2]
Method 1. In a 50 mL polytetrafluoro-lined reactor, add 4 mL of methanol, 0.3 mmol styrene, and 0.1 g of 0.15 wt% Ru/CeO2 catalyst, and add a magnetic sub, filled with 0.5MPa CO, sealed, stirred and reacted at 180°C for 12 hours. After the reaction, the product was characterized by mass spectrometry and quantified by gas chromatography. The product is as follows:
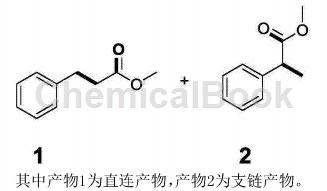
Method 2. Methylation reactions are not only related to biological processes, but also play an important role in the functionalization of biologically active molecules in synthetic chemistry. Traditional methylating reagents include methyl iodide, dimethyl sulfate, methyl p-toluenesulfonate and diazomethane. Although these methylating reagents are efficient, they are often unstable, dangerous or highly toxic. CN201811224832.6 reports that methyl trifluoroacetate is used as a green and environmentally friendly reagent to react in the presence of a base to obtain the corresponding methylated product.
Preparation of 3-phenylpropionic acid methyl ester
Add phenylpropionic acid (100 mg, 0.67 mmol), DMF (4 mL), and potassium tert-butoxide (224 mg, 2.0 mmol, 3eq.) into the reaction flask in sequence, stir at room temperature for 5 minutes, wait until tert-butoxide After the potassium alkoxide is evenly dispersed, slowly inject methyl trifluoroacetate (0.27 mL, 2.7 mmol, 4 eq.) into the reaction bottle. After 4 hours of reaction, the TLC spot plate will show that the raw material point disappears, and the stirring is stopped. Extract twice with ethyl acetate and distilled water, then wash with saturated NaCl solution, combine the organic phases, dry the organic phases over anhydrous magnesium sulfate, filter to remove the magnesium sulfate, rotary evaporate the solvent, and purify by column chromatography to obtain colorless liquid styrene. Acid methyl ester (80 mg, yield 73%). HNMR (400 MHz, CDCl3) δ7.30 (d, = 7.0 Hz, 2H), 7.22 (d, = 7.8 Hz, 3H), 3.70 (s, 3H), 2.98 (t, = 7.8 Hz, 2H), 2.66 (t, = 7.9 Hz, 2H); C NMR (100MHz, CDCl3) δ173.32, 140.54, 128.52, 128.29, 126.29, 51.58, 35.71, 30.97 .
Main reference materials
[1] CN201610943936.7 A method of preparing ester compounds
[2] CN201811224832.6 A method of methylation reaction

 微信扫一扫打赏
微信扫一扫打赏

