background and overview[1]
sulfonate is an anti-coccidial drug belonging to the sulfonamides. this drug inhibits the dihydrofolate synthase in coccidia, hinders folic acid synthesis, blocks nucleic acid synthesis, and inhibits the reproduction of the parasite. the drug is used in coccidia. it inhibits the development of second-generation schizonts during the asexual reproduction stage and also has a certain inhibitory effect on the first-generation schizonts. it is mainly used for the treatment and prevention of poultry coccidiosis. studies have shown that sulfa drugs have a long action time and metabolism in the body. long-term consumption of foods containing such drugs will accumulate in the body, causing harm to human body functions, damaging the human hematopoietic system, and causing hemolytic anemia. and cause pathogens to develop drug resistance and allergic reactions. in order to ensure the safety of consumers, my country and the european union have stipulated that the maximum residue limit (mrl) of sulfonamides in meat products is: 25.0 micrograms/kg for a single sulfonamide, and 100.0 micrograms/kg for the total amount of sulfonamides. at present, there are many literature reports on the detection methods of sulfonamides in animal-derived foods, cosmetics and feeds using modern analytical instruments, mainly including liquid chromatography and liquid chromatography-tandem mass spectrometry. liquid chromatography-tandem mass spectrometry has strong anti-interference ability, good selectivity and high detection sensitivity, and has been widely used in the detection of sulfonamide veterinary drug residues.
preparation[2]
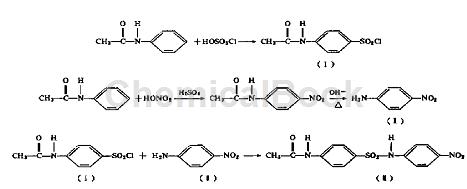
1. preparation of p-acetylfluorobenzenesulfonyl chloride
add 26ml chlorosulfonic acid (0.39m01) into a 250ml dry erlenmeyer flask, bring it to 12~15°c, slowly add 10.6g acetanilide (0.078m01) and heat it to 60°c, and heat for 2 hours. the reactant was moved into 160e crushed ice and stirred. the precipitate was filtered by suction, washed with water and dried to obtain p-acetyl azobenzene sulfonyl chloride [1], which was light pink in color with a yield of 80%.
2. preparation of p-nitroaniline [ii]
add 9ml of concentrated sulfuric acid into a 100rnl beaker, add 3.5g of acetanilide to the sulfuric acid in portions while stirring, and control the temperature below 25°c. after adding, cool it to 0~2°c in an ice bath and add 2ml of concentrated nitric acid dropwise. into acetanilide cold solution and control the reaction below 5°c for 1 hour. slowly move the reaction mixture into a beaker containing 60 ml of ice water. stir and p-nitroacetanilide will crystallize out. filter to obtain the crude product. move the crude product into a cup, add 40ml of water, add sodium carbonate in portions to adjust the ph to l0, heat and boil to hydrolyze o-nitroacetanilide. the mixture was cooled to 50°c, filtered with suction, washed with water, and dried to obtain white crystals of p-nitroacetanilide. add the above crystals into a 100 ml round-bottomed flask, add 25 ml of water and 2.5 ml of 35% sodium hydroxide solution, raise the temperature to 105°c and reflux for 1 hour. cool slightly, move the reactant into a beaker and cool to 40°c to precipitate crystals. filter, wash, and dry to obtain 2.7 g of p-nitroaniline, which is a light yellow needle crystal with a yield of 75.5%.
3. preparation of sulfonate (two synthesis methods)
(1) use n, n-dimethylaniline as solvent
add 12.5rnl anhydrous n, n-dimethylaniline into a 100rnl dry round-bottomed flask and warm it, then add 3.4g p-nitroaniline [ii] (0.025mol) into the flask and wait until it is completely dissolved , add 5.8g acetaminophenyl chloride [1] (0.0025rnol) in portions, and reflux for 1h. after the reaction liquid has cooled n, add 20 ml of 6 mol/l hydrochloric acid and crystals will precipitate. filter, wash and dry to obtain 3.4 g of sulfonamide light green powder with a yield of 40.6%.
(2) use glacial acetic acid as solvent
add 20ml of glacial acetic acid and 3.0g of p-nitroaniline (0.022m01) into a 150ml dry round-bottomed flask. when the temperature reaches 120°c, add 6.3g of acetaminophenyl chloride (0.027mol), reflux and add in portions. anhydrous sodium acetate 2.3g (0.027mol]), react for 1 hour, add 50ml of water, stir, cool, suction filter, wash the crystals with 60% acetic acid, 5mol/l hydrochloric acid, and water, and dry to obtain sulfonamide [1] yellow powder , recrystallized with ethanol to obtain 3.5g of light yellow powder. yield 47.9%.
main reference materials
[1] determination of sulfonate residues in chicken by liquid chromatography and tandem mass spectrometry in negative ion mode
[2] synthesis of the new anti-coccidial drug acesulfonate

 微信扫一扫打赏
微信扫一扫打赏

