Background and overview[1]
4-Chloro-3-hydroxybenzaldehyde can be used as a pharmaceutical synthesis intermediate. If 4-chloro-3-hydroxybenzaldehyde is inhaled, move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eyes If exposed to sunlight, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
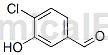
Structure
Preparation[1]
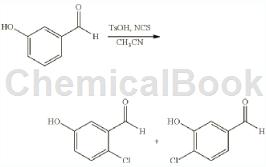
4-Chloro-3-hydroxybenzaldehyde was prepared as follows: To a solution of 3-hydroxybenzaldehyde (1g, 10mmol) in acetonitrile (50mL), p-toluenesulfonic acid (3.4g, 20mmol) was added portionwise. The mixture was stirred at room temperature for 5 minutes, NCS (1.33 g, 10 mmol) was added, and the resulting mixture was stirred at room temperature for 2 hours. The mixture was quenched with aqueous sodium thiosulfate solution and diluted with ethyl acetate and brine. The organic layer was separated, dried and concentrated to obtain crude product, which was purified by silica gel chromatography, eluting with petroleum ether/ethyl acetate (10:1 to 5:1) to obtain 4-chloro-3-hydroxybenzaldehyde ( 310 mg, 19.7%), as a yellow solid.
Application
4-Chloro-3-hydroxybenzaldehyde can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
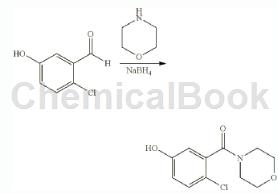
To a mixture of 4-chloro-3-hydroxybenzaldehyde (200 mg, 1.27 mmol, intermediate 28a) and morpholine (280 mg, 3.21 mmol) in methanol (10 mL) was added 2 drops of acetic acid and stirred at room temperature Mixture temperature 2 hours. Sodium borohydride (97 mg, 2.56 mmol) was added to the mixture, and the resulting mixture was stirred at room temperature for 4 hours. The mixture was quenched with dilute hydrochloric acid and concentrated to give crude product. The crude product was purified by silica gel chromatography, eluting with petroleum ether/ethyl acetate (5:1 to 1:1) to give 4-chloro-3-(morpholino)�Methyl)phenol (150 mg, 51.8% yield) as a white solid.
Main reference materials
[1] US20140275528 SUBSTITUTEDXANTHINESANDMETHODSOFUSETHEREOF

 微信扫一扫打赏
微信扫一扫打赏

