Background and overview[1]
Valeric acid is a colorless liquid; it has an unpleasant odor and flavor, similar to butyric acid; melting point is 34℃; boiling point is 184~186℃ (185.5~186.5℃); density D4 200.9459; refractive index nD201.4100; slightly soluble in water, soluble in ethanol and ether, valeric acid can be used to prepare food flavors and Fragrance raw materials. Phenyl valerate is the ester form of valeric acid and can be used as a pharmaceutical synthesis intermediate. If phenyl valerate is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, wash the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if there is eye contact, separate Rinse the eyelids with running water or saline and seek medical attention immediately. If ingested, rinse mouth immediately. Do not induce vomiting and seek medical attention immediately.
Preparation[1]
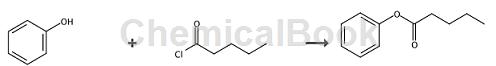
Phenyl valerate is prepared as follows: Dissolve substituted phenol (15mmol) in 20ml of 10% aqueous sodium hydroxide (50mmol NAOH) solution in a 100ml flask. A solution of tetra-n-butylammonium chloride (1.5 mmol) in 5 mL of dichloromethane and an acid chloride (15 mmol) in 15 mL of dichloromethane was prepared. After cooling all solutions at 0°C, they were mixed immediately. The reaction mixture was maintained at 0 °C for 5 min with vigorous magnetic stirring (400 rpm) and then poured into 50 mL of ice water. The organic layer was separated, and the aqueous layer was extracted twice with 40 mL of diethyl ether. The combined organic extracts were washed with saturated NaCl solution. After drying over Na2SO4, the solvent is evaporated and the residue is analyzed directly by gas chromatography (GC), liquid chromatography (LC) and/or GC-mass spectrometry (MS). Quantitative determinations were performed using the internal standard method. The product structure was also identified by infrared (IR), NMR spectroscopy and GC-mass analysis. Amyl valerate yield: 84%.
Main reference materials
[1] O-Acylation of Substituted Phenols with Various Alkanoyl Chlorides Under Phase-Transfer Catalyst Conditions

 微信扫一扫打赏
微信扫一扫打赏

