Background and overview[1]
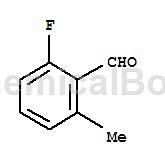
2-Fluoro-6-methylbenzaldehyde can be used as a pharmaceutical synthesis intermediate. If 2-fluoro-6-methylbenzaldehyde is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, wash the skin thoroughly with soap and water, and seek medical treatment if you feel unwell; if In case of eye contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
Apply[1]
2-Fluoro-6-methylbenzaldehyde can be used as a pharmaceutical synthesis intermediate. Using simple and easily available 2-fluoro-6-methylbenzaldehyde: 9,9-dibutyl-2,7-diiodofluorene as the starting material, glacial acetic acid as the solvent, glycine as the temporary directing group, and Palladium acetate was used as the catalyst and silver trifluoroacetate was used as the oxidant. The boiling point of glacial acetic acid is 117°C, so after the reaction is completed, saturated sodium bicarbonate aqueous solution and dichloromethane can be added to extract the organic matter to prepare 15,15-dibutyl-4,9-difluoro-cyclopenta[ 1,2-b: 3,4-b’] dianthracene. The specific reactions are as follows:
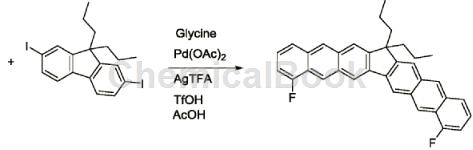
Combine a total of 53 mg (0.1mmol) of 9,9-dibutyl-2,7-diiodofluorene, 34.5mg (0.25mmol) of 2-fluoro-6-methylbenzaldehyde, and 7.5mg (0.1mmol) of glycine. , 2.25 mg of palladium acetate (0.01 mmol), 53.1 mg of silver trifluoroacetate (0.24 mmol) and 15 mg of trifluoromethanesulfonic acid (0.1 mmol) were mixed in 2 ml of glacial acetic acid solvent, and the reaction solution was placed on a magnetic stirrer at 100°C. Stir on top, and after 24 hours of reaction, spot the reaction solution to determine whether the reaction is complete or a certain reactant has reacted completely, and stop the reaction. Add saturated sodium bicarbonate aqueous solution and methylene chloride to the reaction solution for extraction, then remove the lower extract, add an appropriate amount of anhydrous sodium sulfate to dry and remove water, filter and take the filtrate, finally add silica gel powder and spin dry methylene chloride, in which silica gel powder The mass is 30-40 times the mass of the solute in the filtrate. Produced by column chromatography� product, then spin the solvent to dryness, and vacuum to obtain 44.2 mg of condensed ring aromatic hydrocarbons with a benzofluorene structure, with a yield of 86%.
Main reference materials
[1]CN108892601 Preparation method of condensed ring aromatic hydrocarbons with benzofluorene structure

 微信扫一扫打赏
微信扫一扫打赏

