Background and overview[1]
2-Chloro-5-cyanobenzene-1-sulfonyl chloride is a phenyl cyanide derivative and can be used as a pharmaceutical synthesis intermediate.
Structure
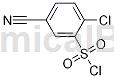
Preparation[1]
The preparation of 2-chloro-5-cyanobenzene-1-sulfonyl chloride is as follows:
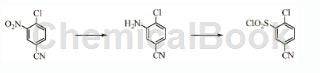
Dissolve commercially available 4-chloro-3-benzonitrile in 4 parts of THF. To a portion of water, add sodium hydrosulfide (3 equiv.) and stir at 45 °C overnight. TLC (25% EtOAc in hexane) confirmed reaction completion. The THF was evaporated in vacuo and the product precipitated. The white solid was collected by vacuum filtration, washed with water and dried in a vacuum oven with gentle heating (97.0% yield). The aniline product was ground in a mortar and pestle and dissolved in a mixture of 11 parts HCl and 4 parts acetic acid. In another container, stir 50 parts of acetic acid with SO2 gas until saturated (confirmed by weight). Place the triacetic acid:aniline mixture in a dry ice/ethanol bath at 10°C. Dissolve sodium nitrite (1.1 equiv) in a minimum amount of water and add dropwise to the HCl salt:aniline mixture without allowing the temperature to rise above about -5°C. The resulting mixture was stirred for 45 minutes to form diazonium ions. Copper (I) chloride (0.1 eq) and copper (II) chloride (0.25 eq) were added to the SO2/acetic acid solution and stirred for 30 minutes and cooled to 10°C in an ice bath. Add the diazo mixture to the copper (I) chloride/copper (II) chloride suspension in batches, keeping the temperature at 30°C or lower. Once completely mixed, stir the mixture until gas evolution stops and a dark green solution forms. Then slowly pour the mixture into 200 parts of ice water with stirring until the ice melts. The resulting white precipitate, 2-chloro-5-cyanobenzene-1-sulfonyl chloride, was collected by vacuum filtration and washed with water (72.9% yield).
Apply[1]
2-Chloro-5-cyanobenzene-1-sulfonyl chloride can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
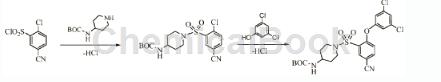
Dissolve 2-chloro-5-cyanobenzene-1-sulfonyl chloride (8.00g) in 100ml THF, and add N-BOC-4-aminopiperidine (5.79g). Slowly equals 6. K2CO3 was added and the mixture was stirred at room temperature for 1 hour. 3,5-Dichlorophenol (11.06g) and 18-crown-6 (4.66g) were added and the reaction was stirred at reflux (75°C) for 4 days. The mixture was cooled and vacuum filtered, and the filtrate was concentrated in vacuo, redissolved in dichloromethane, partitioned with water, and the aqueous layer was extracted three times with dichloromethane. The combined organic layers were washed with saturated sodium bicarbonate, water, brine and dried over MgSO4. The filtrate was concentrated in vacuo, triturated with dichloromethane/diisopropyl ether and vacuum filtered to give a yellow powder. The powder was recrystallized in MeOH to give the product as a white powder (14.50 g, 87.3%).
Main reference materials
[1] WO2011116161 ARYLSULFONAMIDE CCR3 ANTAGONISTS

 微信扫一扫打赏
微信扫一扫打赏

