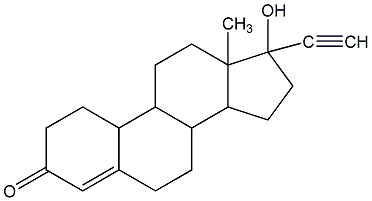
Structural formula
| Business number | 01F8 |
|---|---|
| Molecular formula | C20H26O2 |
| Molecular weight | 298.42 |
| label |
Desmethyldehydrohydroxyprogesterone, norethindrone, 4-19-desmethylandrostenediol, 19-nocetestosterone, Norandrostenone, 19-Norethindrone, 19-Nor-17α-ethynyltestosterone, 17α-Ethynyl-19-nortestosterone |
Numbering system
CAS number:68-22-4
MDL number:MFCD00067596
EINECS number:200-681-6
RTECS number:RC8975000
BRN number:1915671
PubChem number:24897669
Physical property data
1. Properties: White crystalline powder. Odorless and slightly bitter
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain Confirm
4. Melting point (ºC): 205-206 (lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): -31.7 (in chloroform) 10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor Pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Insoluble in water, slightly soluble in ethanol, slightly soluble in ethanol Soluble in acetone, soluble in chloroform
Toxicological data
None
Ecological data
None
Molecular structure data
None
Compute chemical data
None
Properties and stability
A progesterone like progesterone, its effect is 4 times stronger than that of progestin, and its ovulation-inhibiting effect is stronger than that of progesterone. It also has weak androgenic and estrogenic activities.
Storage method
This product should be sealed and stored in a cool, dry place away from light.
Synthesis method
1. Can be synthesized from pregnenolone acetate
2. Preparation method:
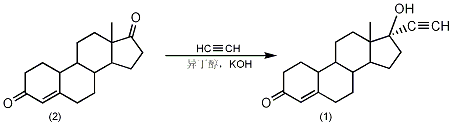
In a reaction flask equipped with a distillation device, add 15g of potassium hydroxide, 120mL of toluene, and 200mL of isobutanol, add nitrogen, and heat to azeotropically dehydrate. Until all the water is removed. Add 100 mL of tetrahydrofuran, control the flow of acetylene gas at around 30°C, and keep the reaction at 25°C for 6 hours (acetylene absorption volume is 200 times). Cool to 0°C and continue to pass acetylene 100 times. Add a solution of 10 g (0.037 mol) of 4-androstene-19-desmethyl-3,17-dione (2) dissolved in 60 mL of tetrahydrofuran, and react for 12 hours below 5°C while continuously feeding acetylene gas. After the reaction is completed, neutralize with 30% sulfuric acid to pH 1-2, and control the reaction temperature not to exceed 40°C. Tetrahydrofuran was evaporated under reduced pressure until solid precipitated, and steam distillation was performed to remove toluene. Cool to below 25°C, filter with suction, wash the filter cake with water until neutral, and dry to obtain crude norethindrone (1). The crude product was washed once with 1.5 times of 95% ethanol, once with 1 time of 70% ethanol, recrystallized with 15 times of 95% ethanol, and decolorized with activated carbon. Filter, recover ethanol from the filtrate, cool, crystallize, filter and dry to obtain fine norethindrone (1), mp 201~208°C, yield 93%. [1]
Purpose
Reference standards for forensic testing. Biochemical research. Organic Synthesis. Contraceptive Preparation.
Progesterone drugs. It mainly promotes and maintains uterine changes in early pregnancy and pregnancy. It is similar to megestrol, but has better hemostatic effect.
It is used for functional uterine bleeding, dysmenorrhea, irregular menstruation, endometriosis and infertility, etc.; it can be used as a contraceptive when combined with estrogen drugs. It is formulated with ethinyl estradiol as contraceptive tablet No. 1 (norethindrone compound tablet).

 微信扫一扫打赏
微信扫一扫打赏

