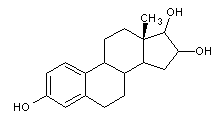
Structural formula
| Business number | 013D |
|---|---|
| Molecular formula | C18H24O3 |
| Molecular weight | 288.38 |
| label |
16,17-dihydroxysterol, 1,3,5(10)-triene-3B,16A,17Btriol, estrogen triol, estriol, (16α,17β)-Estra-1,3,5(10)-triene-3,16,17-triol, E3, 1,3,5(10)-Estratriene-3,16.α.,17.β.-triol, estrogen drugs |
Numbering system
CAS number:50-27-1
MDL number:MFCD00003691
EINECS number:200-022-2
RTECS number:KG8225000
BRN number:2508172
PubChem number:24894396
Physical property data
1. Appearance: white crystalline powder
2. Relative density (g/mL, 25/4℃): 1.27
3. Relative vapor density (g/mL , air=1): Undetermined
4. Melting point (ºC): 280-282 °C (lit.)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2 kPa): Undetermined
7. Refractive index: 58° (C=0.4, Dioxane)
8. Flash Point (ºC): Undetermined
9. Specific optical rotation (º): [α]25D+34.4° (pyridine), 25D+58°±5°(4%, dioxane)
10. Autoignition point or ignition temperature (ºC): Not available Determined
11. Vapor pressure (kPa, 25 ºC): Undetermined
12. Saturated vapor pressure (kPa, 60 ºC): Undetermined
13 . Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Explosion Lower limit (%, V/V): Undetermined
19. Solubility: This crystal is hardly soluble in water, slightly soluble in ethanol (1:500), ether, chloroform, dioxane and vegetable oil , easily soluble in pyridine and alkali hydroxide solutions
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 81.09
2. Molar volume (cm3/mol): 229.6
3. Isotonic specific volume (90.2K ): 622.1
4. Surface tension (dyne/cm): 53.8
5. Polarizability (10-24cm3): 32.14
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 9
6. Topological molecule polar surface area 60.7
7. Number of heavy atoms: 21
8. Surface charge: 0
9. Complexity: 411
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 6
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a dry place away from light.
Synthesis method
1. Estrogen extracted or chemically synthesized from human placental tissue and pregnant women’s urine.
2. Estrone is used as raw material for industrial production.
3.
4. Preparation method:
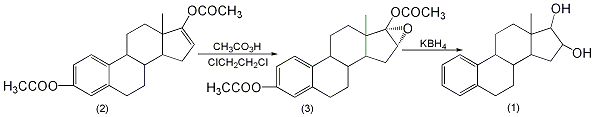
1,2,5(10)-estriol-16a,17a-epoxy-3,17β-diol diacetate (3): reaction equipped with stirrer, thermometer, dropping funnel, and reflux condenser In the bottle, add 10g (28mmol) of 1,3,5(10),16-estraene-3,17-diol diacetate (2), 100mL of dichloroethane, 10g of anhydrous sodium carbonate, anhydrous 13g of sodium sulfate, add dropwise a solution of 27g of peracetic acid (20% to 30%) and 13g of anhydrous sodium acetate while stirring. Control the reaction temperature to not exceed 20°C and complete the addition in about 20 minutes. Continue stirring the reaction for 4 hours. Leave overnight. Filter and wash the filter cake with dichloroethane. Combine the filtrate and washing liquid, wash with sodium bicarbonate aqueous solution, and then wash with water until neutral. Dry over anhydrous sodium sulfate, and evaporate dichloroethane under reduced pressure. Let the crystals settle overnight. Suction filtration, washing with ethanol, and drying were performed to obtain 9.0 g of 1,3,5(10)-estriol-16a,17a-epoxy-3,17β-diol biscerate (3), with a yield of 86%. This triol (1): In a reaction bottle equipped with a stirrer, thermometer, dropping funnel, and reflux condenser, add 5 g of compound (3) and 100 mL of ethanol, and dropwise add 1 g of potassium borohydride and potassium hydroxide under stirring. 4g, 20mL of water to prepare a solution, control the reaction temperature at 25-30°C, complete the addition in about 0.5h, and then continue to stir the reaction for 1h. After the reaction is completed, use glacial acetic acid to make it slightly acidic. Concentrate to dryness under reduced pressure. Add an appropriate amount of water, perform suction filtration, wash the filter cake with water until neutral, and dry it at 80°C to obtain crude estriol. The crude product was purified once with 20 times of 95% ethanol and three times with ethyl acetate to obtain estriol (1) with a yield of 50%. [1]
Purpose
1. Used for cervicitis, especially menopausal syndrome and senile vaginitis.
2. It can be used as an auxiliary drug for mid-term labor induction and artificial abortion.
3. Used for prostatic hypertrophy, prostate cancer, etc.
4. It has the effect of rapidly increasing peripheral leukocytes. It usually takes effect 1 to 3 days after administration, but the effect lasts for a short time. Effective for leukopenia caused by chemotherapy or radiotherapy.
5. It can reduce the permeability and fragility of blood vessels and can be used to treat a variety of bleeding. It has a rapid hemostatic effect on menorrhagia, tonsillectomy or hysterectomy.

 微信扫一扫打赏
微信扫一扫打赏

