Background and overview[1]
Ortho-phthalaldehyde is a pharmaceutical intermediate that has been widely used in amine alkaloids, fluorometer histamine determination and medical testing. The finished product is light yellow needle-shaped crystals, soluble in water, alcohol, ether and other organic solvents, has no bad smell and has stable chemical properties. It was first used for endoscope disinfection in 1994 and was found to have a broad-spectrum and efficient bactericidal effect. Subsequent studies have shown that o-phthalaldehyde has many advantages over glutaraldehyde. As a new type of highly effective chemical disinfectant for external use, phthalaldehyde has passed the US FDA certification in 1999.
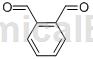
Structure
Bactericidal mechanism[2]
Different from the bactericidal mechanism of general disinfectants, the bactericidal activity of o-phthalaldehyde is based on its cross-linking effect with amino acids and proteins. Studies have shown that o-phthalaldehyde forms a strong cross-linked bond with the bacterial cell wall, forming a barrier that prevents these substances from damaging the cell wall/membrane structure. However, the sealing and anti-leakage effect of o-phthalaldehyde also causes dysfunction in the exchange of substances inside and outside the bacteria, so that the normal physiological functions of the bacteria cannot be carried out, thereby promoting cell death.
On the other hand, the existence form of o-phthalaldehyde in water is different from that of glutaraldehyde. o-phthalaldehyde exists as a single molecule in the solution, so the free aldehyde groups can all exert a disinfection effect, while the dialdehyde molecules are separated by Aldehyde groups exist in the form of polymerization. The acidity and alkalinity of the solution and other factors affect the degree of polymerization. Different degrees of polymerization result in different numbers of exposed free aldehyde groups. The number of free aldehyde groups determines its disinfection effect. Therefore, the sterilization effect of o-phthalaldehyde is better than that of glutaraldehyde. . Since o-phthalaldehyde is an aromatic aldehyde, it has good lipid solubility and can easily penetrate the cell membranes of Mycobacterium tuberculosis and Gram-negative bacteria with more lipids, thereby acting on target sites inside the bacteria and causing rapid cell death. Phthalaldehyde has a stronger killing effect on Mycobacterium tuberculosis and Gram-negative bacteria than glutaraldehyde.
Spores are more resistant to the outside world than bacterial propagules. This is because an outer structure containing a large amount of picolinic acid is formed in the later stages of spore maturation. Research has found that o-phthalaldehyde mainly interacts with the spore shell. The cross-linking of amino acids causes the death of spores. This cross-linking forms a lethal and strong coat that makes the spores unable to germinate. Therefore, o-phthalaldehyde kills Bacillus subtilis spores not by damaging the DNA, but by causing the spores to germinate. Inactivation occurs and o-phthalaldehyde irreversibly damages the spore germination process.
Stability and corrosiveness[2]
O-phthalaldehyde has the characteristics of stable performance compared with glutaraldehyde. The content of 5969 mg/L o-phthalaldehyde dropped to 5776 mg/L after being stored at 54°C for 14 days, with an average decrease rate of 3.23%; after being stored at 37°C for 90 days, the content dropped to 5672 mg/L, with an average decrease rate of 4.98%. In the test, it was found that the concentration of 5000 mg/L o-phthalaldehyde remained above 4500 mg/L after 14 days of continuous use, exceeding the 14 days of continuous use. At the same time, some data show that when an automatic endoscope cleaning machine is used to disinfect endoscopes, o-phthalaldehyde reaches the minimum effective concentration after being reused 82 times, while glutaraldehyde can only be re-disinfected 40 times.
It shows that the continuous use period of glutaraldehyde is relatively short. In the metal corrosion test, stainless steel, carbon steel, copper, and aluminum sheets were soaked with 5000 mg/L o-phthalaldehyde for 72 hours. The average corrosion rates were 0.000, 0.003, 0.102, and 0.006rnln/a, indicating that o-phthalaldehyde removes Except for slight corrosion on copper metal, it has no corrosion on other metals and has wide material compatibility.
Apply[2]
O-phthalaldehyde has a strong killing effect on bacterial propagules, fungi, mycobacteria, viruses, and spores. Experiments were conducted on the effect of o-phthalaldehyde on killing spores of Bacillus subtilis var. black (C9372), Candida albicans (AT0C10231), Escherichia coli (8099), Staphylococcus aureus (ATCC6538) and Pseudomonas aeruginosa PA01. In the carrier sterilization test, 5000 mg/L o-phthalaldehyde acted on Escherichia coli and Staphylococcus aureus for 3 minutes, and on Candida albicans for 5 minutes, resulting in a reduction of more than 6 log of the three bacteria.
In the suspension test, 18rng/L o-phthalaldehyde solution was used for 20 minutes.Can kill more than 5log of Pseudomonas aeruginosa. When the o-phthalaldehyde concentration is 180mg/L for 5 minutes, Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa can all be reduced by more than 5log, while glutaraldehyde requires 450mg/L to achieve the same killing effect. However, o-phthalaldehyde has relatively weak sporicidal ability under neutral conditions. In the sporicidal test, the killing logarithm of 5000mg/L Bacillus spores was only 0.09; but when the pH was adjusted to 8, 4959mg/L o-phthalaldehyde was used for 240 minutes and 5969mg/L o-phthalaldehyde was used for 120 minutes. , the killing logarithms of Bacillus subtilis spores on stainless steel carriers reached 3.69 and 5.0 respectively, indicating that the killing effect of o-phthalaldehyde on dental cells is significantly improved under alkaline conditions.
It has also been found that o-phthalaldehyde has a rapid killing effect on mycobacteria, especially glutaraldehyde-resistant Mycobacterium chelonae. Phthalaldehyde at a concentration of 5000 mg/L was used for 10 min and 20 min respectively to kill two glutaraldehyde-resistant strains of Epping Mycobacterium chelonae and Hardidd Mycobacterium chelonae, with a killing effect of more than 6 log. Using 2100 mg/L phthalaldehyde, When formaldehyde acts for 5 minutes, the killing effect on Mycobacterium tuberculosis can reach more than 5 log, while 15000 mg/L glutaraldehyde needs to act on these Mycobacterium chelonae for 32 rnin to achieve the same killing effect.
The U.S. Environmental Protection Agency has identified duck HBV as an indicator strain for inactivation of human HBV. An experiment using o-phthalaldehyde to inactivate duck HBV vector showed that 3000mg/L o-phthalaldehyde can effectively kill duck hepatitis B virus at 20°C for 5 minutes.
Preparation[3]
Green preparation process of o-phthalaldehyde:
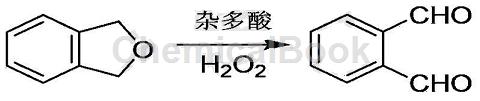
1) Add 0.1 to 0.3 mol of 1,3-dihydroisobenzofuran, 0.2 to 0.6 mol of oxidant, 0.3 to 0.5 g of catalyst heteropolyacid, and 10 to 30 g of water in a three-necked flask;
2) Add an electromagnetic stirrer to the above three-necked flask, install a reflux condenser and a temperature probe, and perform an ultrasonic stirring reaction; set the reaction temperature to 30 to 60°C, the ultrasonic power to 200 to 500W, and the reaction running time 30min~180min;
3) After the reaction is completed, lower to room temperature, use an equal volume of ethyl acetate to extract three times, combine the extracts, and remove the ethyl acetate by distillation to obtain crude o-phthalaldehyde;
4) Mix the crude o-phthalaldehyde and the recrystallization solvent petroleum ether at a mass ratio of 1:3 and then recrystallize. Filter to obtain o-phthalaldehyde crystals. Place the o-phthalaldehyde crystals in a vacuum with a degree of Dry in a vacuum drying oven at 60Pa and 50℃ for 4 hours to obtain o-phthalaldehyde product;
5) Use a rotary evaporator to recover the petroleum ether obtained in step 4. The recovered petroleum ether can be reused.
Main reference materials
[1] Research on factors affecting the bactericidal effect of o-phthalaldehyde
[2] Research progress on o-phthalaldehyde disinfectants
[3] Green preparation process of CN201310242491.6 o-phthalaldehyde

 微信扫一扫打赏
微信扫一扫打赏

