Background and overview[1][2]
2,4,6-Trimethylbenzenesulfonyl chloride is an organic chemical intermediate that can undergo acylation reactions with some nucleophilic groups and can also be used as an activator to synthesize cinpazide maleate.
Apply[1-2]
1. Used in the synthesis of cinpazide maleate
Cinepazide maleate is a calcium channel blocker. By preventing Ca2+ from crossing the membrane into vascular smooth muscle cells, it relaxes vascular smooth muscles and affects cerebral blood vessels, coronary blood vessels and peripheral blood vessels. Blood vessels dilate, thereby relieving vasospasm, reducing vascular resistance, and increasing blood flow. It can enhance the effects of adenosine and cyclic adenosine monophosphate (cAMP) and reduce oxygen consumption. This product can inhibit cAMP phosphodiesterase and increase the amount of cAMP. It can also improve the flexibility and deformability of red blood cells, improve their ability to pass through small blood vessels, reduce the viscosity of blood, and improve microcirculation. This product promotes and improves brain metabolism by increasing blood flow in cerebral blood vessels.
CN200910010910.7 provides a synthesis method of cinpazide maleate, which belongs to the field of medical technology. It is characterized by using trans-3,4,5-trimethoxycinnamic acid as raw material, using a carboxyl activator under the action of an organic base to synthesize cinepazide free base through a “one-pot cooking” method. The activators used are alkyl chloroformate, methylsulfonyl chloride, benzenesulfonyl chloride, p-methylsulfonyl chloride, diphenylphosphinate chloride, 2,4,6-trimethylbenzenesulfonyl chloride, N,N-di Any one of cyclohexylcarbodiimide (DCC) and carbonyldiimidazole (CDI); preferably ethyl chloroformate, the reaction temperature is -10°C, and the solvent is dichloromethane. The obtained free base is converted into a salt to obtain cinepazide maleate. The effects and benefits of the present invention are that it simplifies the production conditions and processes, avoids the application of the active intermediate trans-3,4,5-trimethoxycinnamoyl chloride, reduces environmental hazards, and lowers equipment requirements; and provides a new , simple and easy to control, mild reaction conditions, environmentally friendly and suitable for industrial-scale production.
2. Used to synthesize a disubstituted double-positive center 6-alkylimidazolium-6-ammonium-β-cyclodextrin
As chiral resolving agents, cyclodextrin and its derivatives have received widespread attention and extensive research. So far, many negatively charged cyclodextrins have been developed, whereas positively charged cyclodextrins have been reported less frequently. In recent years, there have been more and more studies on positively charged cyclodextrins. The monosubstituted single positively charged cyclodextrin was first reported by Terabe in 1989. Its application in capillary electrophoresis chiral separation of drugs has attracted the attention of researchers. extensive attention. Compared with neutrally charged cyclodextrins, positively charged cyclodextrins have better water solubility and can achieve faster resolution of various racemic drugs at lower concentrations.
CN201110118488.4 provides a method for preparing disubstituted double-positive center 6-alkylimidazolium-6-ammonium-β-cyclodextrin using 2,4,6-trimethylbenzenesulfonyl chloride. Method, the general structural formula is:
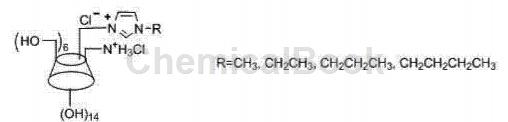
Includes the following steps:
In the first step, p-toluenesulfonyl chloride and imidazole undergo a nucleophilic substitution reaction in the solvent to obtain the product p-toluenesulfonylimidazole;
In the second step, place p-toluenesulfonyl imidazole, the product of the first step, in an aqueous solution in which β-cyclodextrin is dissolved, add sodium hydroxide solution after stirring, filter the filtrate and add ammonium chloride to adjust the pH value to obtain Product 6-p-toluenesulfonyl-β-cyclodextrin Ts-CD, vacuum dry the product;
The third step is to dissolve the product Ts-CD of the second step in deionized water, then add sodium azide to the solution, stir and reflux, concentrate the solution, and drop the concentrated solution into 1,1,2,2 -Precipitate a solid in tetrachloroethane and dry it under vacuum to obtain 6-azido-β-cyclodextrin;
The fourth step is to dissolve the product of the third step, 6-azido-β-cyclodextrin, in pyridine, add 2,4,6-trimethylbenzenesulfonyl chloride, stir, and add the solution to acetone after the reaction. The solid is precipitated, washed with acetone, filtered and dried to obtain the product 6-azido-6-(2,4,6-trimethylbenzenesulfonyl)-β-cyclodextrin;
In the fifth step, evacuate the closed reaction device first and then vent nitrogen, and paste the product of the fourth step, 6-azido-6-(2,4,6-trimethylbenzenesulfonyl)-β-cyclopaste Essence and N,N-dimethylformamide—DMF, were added to the reaction vessel in sequence, then alkyl imidazole was added to the mixed solution, and stirred.Reflux, after the reaction the solution is added to acetone, the solid is precipitated, washed with acetone, filtered and dried to obtain the product 6-azido-6-alkylimidazolium-β-cyclodextrin;
The sixth step is to dissolve the product of the fifth step, 6-azido-6-alkylimidazolium-β-cyclodextrin, in DMF, then add triphenylphosphine to the solution, stir, and wait for a period of time. , add water to the reaction solution, then stir and reflux, finally add the reacted solution to acetone, precipitate the solid, wash with acetone, filter and dry to obtain the product 6-amino-6-alkylimidazolium-β-ring Dextrin;
The seventh step is to dissolve the product of the sixth step, 6-amino-6-alkylimidazolium-β-cyclodextrin, in a dilute hydrochloric acid solution, stir, and concentrate the solution. The concentrated solution is dropped into acetone to precipitate a solid. , filter and dry; then dissolve the dry solid in deionized water, add anion exchange resin, let it stand for filtration, add the filtrate to acetone, precipitate the solid, filter and dry, and obtain the product: Cl- is an anion, double-substituted double-positive center 6 -Ammonium 6-alkylimidazolium-β-cyclodextrin.
Compared with the existing technology, the present invention has significant advantages: (1) Disubstituted double-positive center-β-cyclodextrin has ammonium group and imidazolium salt group, so it has more advantages than β-cyclodextrin. Better water solubility; (2) Disubstituted double positive center-β-cyclodextrin, its ammonium substituent gives it excellent resolving power for acidic compounds; (3) Disubstituted double positive center-β -The amine group and onium salt of cyclodextrin can dissociate by themselves, so that during the resolution process, its positive charge is not affected by the pH of the buffer; (4) The imidazolium group makes it resistant to amino acid racemates It has excellent resolving power. The ammonium group makes it have excellent resolving power for acidic compounds. Therefore, the disubstituted double-positive center-β-cyclodextrin is expected to simultaneously achieve the resolving power of dansyl amino acids, α-hydroxy acids and carboxylic acids. A variety of racemic drugs can achieve efficient resolution over a wider pH range.
Main reference materials
[1] CN200910010910.7 A synthesis method of cinpazide maleate
[2] CN201110118488.4 A disubstituted double-positive center 6-alkylimidazolium-6-ammonium-β-cyclodextrin and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

