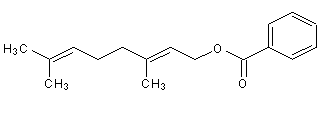
Structural formula
| Business number | 027R |
|---|---|
| Molecular formula | C17H22O2 |
| Molecular weight | 258.36 |
| label |
(E)-3,7-Dimethyl-2,6-octadien-1-ol benzoate |
Numbering system
CAS number:94-48-4
MDL number:MFCD00036513
EINECS number:202-337-0
RTECS number:RG5925300
BRN number:None
PubChem number:24900979
Physical property data
1. Characteristics: Colorless to slightly yellow liquid. It has the aroma of ylang-ylang oil, and it has the aroma of apple after being highly diluted.
2. Density (g/mL,20℃): 0.996
3. relative Vapor Density (g/mL,Air =1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC,normal pressure):305
6. Boiling point (ºC,0.933KPa):127~129
7. Refractive index:1.517
8. Flashpoint (ºC):>100
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,25ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of the partition coefficient for water: undetermined
Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,25ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of the partition coefficient for water: undetermined
17. Explosion upper limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility:Insoluble in water. Soluble in oil and alcohol.
Toxicological data
1, Acute toxicity: Rat oral LD50:>5mg/kg; Rabbit skin contactLD50:>5mg/kg;
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 5.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 329
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
In pyridine solution, it is made by reacting geraniol with benzoyl chloride; or it is catalyzed by sulfonic acid camphor to make geraniol and benzoyl Derived from function.
Purpose
Spice. It is mainly used to prepare fruit-based flavors such as apples, and is also used as a fixative and coordinating agent for rose-based flavors.
evel1 lfo1; tab-stops: list 36.0pt; mso-pagination: widow-orphan; mso-margin-top-alt: auto; mso-margin-bottom-alt: auto” align=left>17. Explosion upper limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility:Insoluble in water. Soluble in oil and alcohol.
Toxicological data
1, Acute toxicity: Rat oral LD50:>5mg/kg; Rabbit skin contactLD50:>5mg/kg;
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 5.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 329
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
In pyridine solution, it is made by reacting geraniol with benzoyl chloride; or it is catalyzed by sulfonic acid camphor to make geraniol and benzoyl Derived from function.
Purpose
Spice. It is mainly used to prepare fruit-based flavors such as apples, and is also used as a fixative and coordinating agent for rose-based flavors.

 微信扫一扫打赏
微信扫一扫打赏

