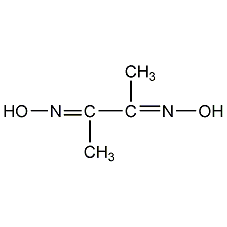
Structural formula
| Business number | 0292 |
|---|---|
| Molecular formula | C4H8N2O2 |
| Molecular weight | 116.12 |
| label |
diacetyl oxime, nickel reagent, diacetyl oxime, dimethylglyoxime, 2,3-butanedionedioxime, 2,3-diisonitrosobutane, Diacetyldioxime, (CH3)2C2(NOH)2, 2,3-diisonitrosobutane, Diacetyldioxime, 2,3-Butandion dioxime, color developer, extraction agent |
Numbering system
CAS number:95-45-4
MDL number:MFCD00002117
EINECS number:202-420-1
RTECS number:EK2975000
BRN number:506731
PubChem number:24865222
Physical property data
1. Properties: White triclinic crystal or crystalline powder.
2. Density (g/mL, 20℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 238~240
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, KPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, ether, acetone and pyridine, almost Insoluble in water.
Toxicological data
1. Acute toxicity: rat oral LDLo: 250mg/kg;
2. Mutagenicity
Hamster embryo morphological transformation: 100μg/L;
Ecological data
Slightly harmful to water.
Molecular structure data
1. Molar refractive index: 28.41
2. Molar volume (cm3/mol): 98.8
3. Isotonic specific volume (90.2K ): 244.6
4. �Surface tension (dyne/cm): 37.5
5. Polarizability (10-24cm3): 11.26
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0.7
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 4
6. Topological molecular polar surface area (TPSA): 61.7
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 119
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides.
Storage method
1. Store sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep sealed.
2. Keep away from fire sources and store away from oxidants and acidic substances.
Synthesis method
1. Obtained from the reaction of diacetyl oxime and hydroxylamine-sodium sulfonate. Add diacetyl oxime to the hydroxylamine-sodium sulfonate solution, heat to 70°C, and keep warm for several hours to precipitate diacetyl oxime crystals. After cooling, filter immediately and wash with ice water until no more sulfate is contained. , that’s it. Hydroxylamine-sodium sulfonate can be prepared as follows: mix sodium nitrite with crushed ice, add a suspension of sodium bisulfite and water while stirring, then add glacial acetic acid from below the liquid surface while stirring, and then add Add concentrated hydrochloric acid and crushed ice to keep the reaction solution below 0°C. Use Congo red test paper to test for an acidic reaction. Filter to remove the insoluble precipitate to obtain a hydroxylamine-sodium sulfonate solution.
2.Add 70% sodium bisulfite suspension to 10% sodium nitrite ice water below 0°C under stirring In the mixture, stir continuously, add glacial acetic acid under the liquid surface, and then add a mixture of concentrated hydrochloric acid and crushed ice. During the reaction, add ice to control the temperature not to exceed 0°C. After the reaction, the solution is acidic to Congo red:
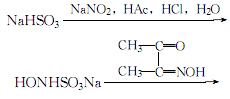
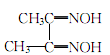
Filter out the insoluble matter to obtain an acidic solution of sodium hydroxysulfamate. Add diacetyl oxime to the above acidic solution, stir and heat to 70-80°C, maintain for more than 2 hours, let it stand, and filter after the crystallization is complete. The crystals are washed with water until neutral, dried, dissolved in ethanol, and then added with an appropriate amount of activated carbon for decolorization, evaporated, concentrated, and dried to obtain white dimethylglyoxime crystals.
Purpose
1. Used for the verification and determination of nickel. Separate nickel from cobalt and other metals, and separate palladium from tin, gold, rhenium, iridium, etc. Photometric determination of cyanide, nickel, and palladium.
2.Spectrophotometric method for determination of chromogenic reagents such as nickel, weighing method for determination or precipitation separation of Ni2+ , Pd2+ and other precipitants. It can also be used as an extraction agent.

 微信扫一扫打赏
微信扫一扫打赏

