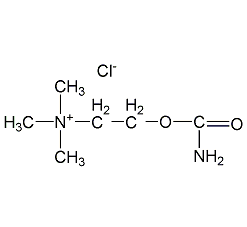
Structural formula
| Business number | 015A |
|---|---|
| Molecular formula | C6H15ClN2O2 |
| Molecular weight | 182.65 |
| label |
carbamylcholine, Carbamylcholine hydrochloride, carbachol, Ammonium Chloride Formylcholine, Hydroxyurea, chromatogram, Kabadin, Capco, chloroacylcholine, choline, Carbamylcholine chloride, Carbamylcholine chloride, carbamylcholine chloride, Carbamoylcholine chloride, (2-Hydroxyethyl)trimethylammonium chloride carbamate |
Numbering system
CAS number:51-83-2
MDL number:MFCD00012011
EINECS number:200-127-3
RTECS number:GA0875000
BRN number:3917459
PubChem number:24277829
Physical property data
1. Character:It is a white crystalline powder, hygroscopic, odorless, and has a faint smell similar to fatty amines.
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC): 204-205
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive index: undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º):Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. 16. oil and water Alcohol/Water) distribution Log value of coefficient: Undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility: Soluble in water, ethanol, methanol, insoluble in acetone, ether or chloroform.
Toxicological data
None
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 2
6. Topological molecule polar surface area 52.3
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 117
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
If placed in an open container, it will produce a slight odor of fatty amines. Hygroscopic.
Storage method
Should be sealed and stored in a dry place.
Synthesis method
Withβ-Chlorohydrin is used as raw material and acylated with phosgene to prepare chloroformic acid-β-Ethyl chloride: Then reacts with ammonia to form carbamic acid-β-Ethyl chloride, and finally reacts with trimethylamine to form a quaternary ammonium salt to obtain kappa Choline: Another method of preparing carbachol is to use choline chloride and urea as raw materials: when the ingredients are used, the ratio of choline chloride: urea: sodium nitrite: sulfuric acid=1:1:1.15:1.35, in60℃Response about40h, can be used as 71%The yield of carbachol was obtained.
Purpose
This product is a cholinergic drug and is also used as a parasympathetic stimulant. When used as veterinary medicine, it is used in large animals to treat acute abdominal pain in horses.
ILY: 宋体; mso-ascii-font-family: Arial; mso-hansi-font-family: Arial; mso-bidi-font-family: Arial”>If placed in an open container, it will produce a slight odor of fatty amines. Hygroscopic.
Storage method
Should be sealed and stored in a dry place.
Synthesis method
Withβ-Chlorohydrin is used as raw material and acylated with phosgene to prepare chloroformic acid-β-Ethyl chloride: Then reacts with ammonia to form carbamic acid-β-Ethyl chloride, and finally reacts with trimethylamine to form a quaternary ammonium salt to obtain kappa Choline: Another method of preparing carbachol is to use choline chloride and urea as raw materials: when the ingredients are used, the ratio of choline chloride: urea: sodium nitrite: sulfuric acid=1:1:1.15:1.35, in60℃Response about40h, can be used as 71%The yield of carbachol was obtained.
Purpose
This product is a cholinergic drug and is also used as a parasympathetic stimulant. When used as veterinary medicine, it is used in large animals to treat acute abdominal pain in horses.

 微信扫一扫打赏
微信扫一扫打赏

