Background and overview[1]
6-Hydroxyanthranilic acid is a carboxylic acid derivative and can be used as a pharmaceutical synthesis intermediate. If 6-hydroxyanthranilic acid is inhaled, please move the patient to fresh air; if skin contact occurs, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if contact with eyes , you should separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
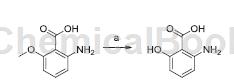
To a solution of 2-amino-6-methoxybenzoic acid (1 equiv) in anhydrous CH2Cl2 at 78°C (dry ice/acetone bath), add boron tribromide (1M in CH2Cl2, dropwise Add 3 equivalents). The reaction mixture was stirred at room temperature under nitrogen atmosphere for 18 hours. Then EtOH and MeOH were added, and the solvent was evaporated to obtain 6-hydroxyanthranilic acid as a brown solid solvent, yield: 75%.
Apply[1]
6-Hydroxyanthranilic acid can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
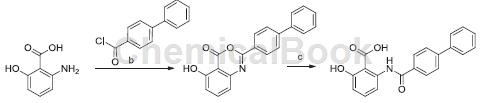
1) Use 6-hydroxyanthranilic acid and intermediate b as reaction raw materials, add a solvent, evaporate the solvent, and suspend the remaining solid in methanol. After filtration, the precipitate was washed with MeOH to provide the title compound (80%). The beige solid was used in the next reaction without further purification; beige solid, yield: 50%.
2) Combine the compound 2-([1,10-biphenyl]-4-yl)-5-hydroxy-4H-benzo[d][1,3]oxazin-4-one obtained in step 1) Dissolve in THF and hydrolyze with aqueous solution containing 1MLiOH at room temperature (18 hours). The mixture was acidified by adding 1 M HCl, filtered, and the precipitate was washed continuously and extracted with 1 M HCl and MeOH to obtain the title compound. Brown solid, yield: 61%.
Main reference materials
[1] Benzamidobenzoic acids as potent PqsD inhibitors for the treatment of Pseudomonas aeruginosa infections

 微信扫一扫打赏
微信扫一扫打赏

