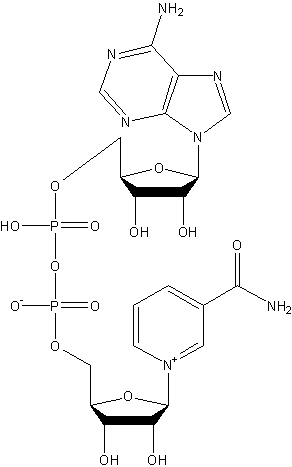
Structural formula
| Business number | 0162 |
|---|---|
| Molecular formula | C21H27N7O14P2 |
| Molecular weight | 663.43 |
| label |
nicotinamide adenine dinucleotide, nicotinamide adenine dinucleotide, Coenzyme I |
Numbering system
CAS number:53-84-9
MDL number:MFCD00036253
EINECS number:200-184-4
RTECS number:UU3450000
BRN number:3584133
PubChem number:24897862
Physical property data
1. Character:White powder. Very hygroscopic.
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC): 266
5. Boiling point (ºC,Normal pressure):524
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive index: undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º):[α]D 23-34.8°(C=1, water)
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC8. Surface charge: 0
9. Complexity: 1120
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 8
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
at260 It has the maximum ultraviolet absorption spectrum at nanometers. Through various deaminase enzymes, it accepts a hydrogen atom and an electron from the substrate and becomes a reduced form. E′0=-0.32V. At this time, the pyridine ring is reduced, and since there is maximum absorption at 340 nanometers, the progress of the reaction can be measured. This reaction can also proceed reversibly. Therefore NAD+ can be used as a common substrate for various deaminase enzymes, but it acts between two dehydrogenases, and its existence in trace amounts can catalyze both substrates. Oxidation-reduction reactions (electron transfer) between substances.
Storage method
This product should be sealed in4Preserve dry.
Synthesis method
None
Purpose
Biochemical research. Medical research, clinical diagnosis. Organic Synthesis. Participates in energy metabolism and material metabolism in the body, which is beneficial to cell repair and renewal. Used for the treatment of coronary heart disease, myocarditis, and leukopenia.
PAN> can be used as a common substrate for various deaminase, but it acts between two dehydrogenases. Its existence in trace amounts can catalyze the redox reaction (electron transfer) between the two substrates.
Storage method
This product should be sealed in4Preserve dry.
Synthesis method
None
Purpose
Biochemical research. Medical research, clinical diagnosis. Organic Synthesis. Participates in energy metabolism and material metabolism in the body, which is beneficial to cell repair and renewal. Used for the treatment of coronary heart disease, myocarditis, and leukopenia.

 微信扫一扫打赏
微信扫一扫打赏

