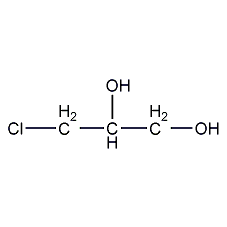
Structural formula
| Business number | 02AT |
|---|---|
| Molecular formula | C3H7ClO2 |
| Molecular weight | 110.54 |
| label |
α-chloropropanediol, α-Chloroglycerol, α-chloroglycerin, α-Chlorohydrin, α-Glycerol chlorohydrin, α-Monochlorohydrin, Solvents for cellulose acetate, etc., dye intermediates, alcohol compounds |
Numbering system
CAS number:96-24-2
MDL number:MFCD00004712
EINECS number:202-492-4
RTECS number:TY4025000
BRN number:635684
PubChem number:24892264
Physical property data
1. Properties: colorless liquid, which gradually turns into a slightly greenish yellow liquid with a pleasant smell after being placed.
2. Relative density (g/mL, 20/4℃): 1.3204
3. Boiling point (ºC, decomposition): 213
4. Boiling point (ºC, 1.466kpa): 116
5. Melting point (ºC): -40
6. Refractive index (n20ºC): 1.4809
7. Viscosity (mPa·s, 20ºC): 159
8. Flash point (ºC, opening): 137.8
9. Heat of combustion (KJ/mol, 20ºC): 1680.6
10. Vapor pressure (kPa, 141ºC): 5.33
11. Vapor pressure (kPa, 113ºC): 1.33
12. Vapor pressure (kPa, 83ºC): 0.133
13. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -525.3
14. Solubility: soluble in water , ethanol, ether and acetone. Slightly soluble in toluene, insoluble in benzene, petroleum ether and carbon tetrachloride.
Toxicological data
1. Acute toxicity: Rat oral LD50: 150mg/kg, mouse oral LD50: 150mg/kg, rat inhalation MLC is 125mg/m3
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 23.82
2. Molar volume (cm3/mol): 84.8
3. Isotonic specific volume (90.2K ): 220.7
4. Surface tension (dyne/cm): 45.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 9.44
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.5
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
p>
4. Rotatable chemical bondsNumber: 2
5. Number of tautomers: None
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 32
10. Number of isotope atoms: 0
11. Determine the atoms Number of stereocenters: 0
12. Uncertain number of stereocenters of atoms: 1
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid exposure to light, moist air, and contact with strong oxidants and strong alkali. It is hygroscopic and will gradually turn yellow when exposed to light or left for a long time. Gradually decomposes into acid when exposed to moisture for a long time.
Chemical properties: 3-chloro-1,2-propanediol partially decomposes when heated to 140~142°C. Oxidation with concentrated nitric acid produces 3-chlorolactic acid. Oxidation with potassium periodate in dilute nitric acid solution produces chloroacetaldehyde. 3-Chloro-1,2-propanediol is dehydrochlorinated to form glycidol. When refluxing with water for a long time, it gradually hydrolyzes to form glycerol. Under the action of alkoxy metal compounds equivalent to primary alcohols, glycerol-α-alkyl ether is generated.
2. Exist in smoke.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from chlorination of glycerol. Add glycerin and acetic acid to the reaction pot, stir and heat to 90-95°C, and pass dry hydrogen chloride gas until the weight of the reaction solution increases to 150% (theoretical value), which is the end point. Distill under reduced pressure to obtain crude product. Fractionate again and collect the 128-132°C (1.33-2.67kPa) fraction to obtain 3-chloro-1,2-propanediol.
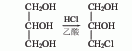
2. From epichlorohydrin Derived from hydrolysis. Add epichlorohydrin into the reaction pot, stir, add dilute sulfuric acid to make the reaction solution acidic, raise the temperature, reflux for 1 hour, and distill the product under reduced pressure to obtain the finished product. Raw material consumption quota: epichlorohydrin (>95%) 1200kg/t, sulfuric acid 3kg/t.
![]()
Refining method: Refined by vacuum distillation .
3. Preparation method:
![]()
In a reaction bottle equipped with a stirrer, thermometer, dropping funnel, and reflux condenser, add 120 mL of water and 1 mL of concentrated sulfuric acid, add to 80°C, and slowly add epichlorohydrin ① (2) 120g (1.3mol), after adding, continue to stir the reaction at 90°C for 1.5h to complete the reaction. Concentrate under reduced pressure to remove the water, and finally collect the fraction at 137-140°C/2.4kPa to obtain 100g of 3-chloro-1,2-propanediol, with a yield of 70%. Note: ① The hydrolysis of epichlorohydrin is an exothermic reaction, and the dripping speed should not be too fast, otherwise the reactants will rush out of the reaction bottle due to violent reaction. [1]
Purpose
It is used in organic synthesis, as an intermediate for drugs, as a solvent for cellulose acetate, etc., and for the preparation of plasticizers, surfactants, dyes, drugs, and glycerin derivatives. In addition, it can also be used to lower the freezing point of yellow explosives.

 微信扫一扫打赏
微信扫一扫打赏

