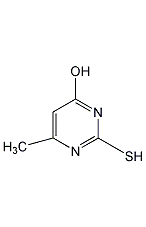
Structural formula
| Business number | 0173 |
|---|---|
| Molecular formula | C5H6N2OS |
| Molecular weight | 142.18 |
| label |
6-Methyl-2-thiouracil, MZU |
Numbering system
CAS number:56-04-2
MDL number:MFCD00006040
EINECS number:200-252-3
RTECS number:YR0875000
BRN number:115648
PubChem number:24895573
Physical property data
1. Character:White crystal. in326-331℃ decomposition. Saturated aqueous solution is neutral or slightly acidic to litmus. Odorless, bitter, easy to sublime.
2. Density (g/mL,25/4℃): Unknown OK
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC):330℃
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC, 1.60kPa): Undetermined.
7. Refractive index: Undetermined
8. Flash Point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC<SPAN style="FONT-SIZE: 9pt; FONT-FAMILY: 宋体; mso-bidi-font-family: Arial; mso-font-kerning: 0pt; mso-ascii-font- family: Arial; ms2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 6
6. Topological molecular polar surface area (TPSA):41.1
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 197
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
It is derived from the reaction of ethyl acetoacetate and thiourea. Dissolve thiourea in sodium hydroxide solution, add ethyl acetoacetate dropwise, and control the temperature at 37℃below. After reacting 2h, add water and activated carbon, stir at 70-80℃Decolorization 0.5h. Filter, acidify the filtrate with hydrochloric acid to pH4-5, cool to room temperature, filter and wash. The filter cake is recrystallized with water to obtain the finished product. The yield is 66%. During production, ethyl acetoacetate and thiourea can also be mixed first, and then sodium oxide solution is added dropwise; anhydrous sodium carbonate can also be used instead of sodium hydroxide, and the yield is roughly the same.
Purpose
Intermediate of cardiovascular drug dipyridamole
mes New Roman'”> Complexity: 197
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
It is derived from the reaction of ethyl acetoacetate and thiourea. Dissolve thiourea in sodium hydroxide solution, add ethyl acetoacetate dropwise, and control the temperature at 37℃below. After reacting 2h, add water and activated carbon, stir at 70-80℃Decolorization 0.5h. Filter, acidify the filtrate with hydrochloric acid to pH4-5, cool to room temperature, filter and wash. The filter cake is recrystallized with water to obtain the finished product. The yield is 66%. During production, ethyl acetoacetate and thiourea can also be mixed first, and then sodium oxide solution is added dropwise; anhydrous sodium carbonate can also be used instead of sodium hydroxide, and the yield is roughly the same.
Purpose
Intermediate of cardiovascular drug dipyridamole

 微信扫一扫打赏
微信扫一扫打赏

