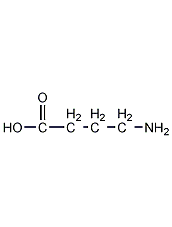
Structural formula
| Business number | 0178 |
|---|---|
| Molecular formula | C4H9NO2 |
| Molecular weight | 103.12 |
| label |
Gamma-aminobutyric acid, γ-Aminobutyric acid, 4-Aminobutanoic acid, GABA, Piperidic acid, amino acid drugs, intermediates, Biochemical reagents |
Numbering system
CAS number:56-12-2
MDL number:MFCD00008226
EINECS number:200-258-6
RTECS number:ES6300000
BRN number:906818
PubChem number:24891025
Physical property data
1. Character: flake or needle crystal.
2. Density (g/mL, 25/4℃): 1.11
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 202 (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, insoluble in ethanol and ether and benzene.
Toxicological data
1. Acute toxicity: rat abdominal LD50: 5400mg/kg; rat brain LDLo: 18mg/kg; mouse oral LD50: 12680mg/kg; mouse abdominal LC50: 4950mg/kg; mouse subcutaneous LC50: 9210mg/ kg; mouse intravenous LC50: 2748mg/kg; mouse LC50: 7230mg/kg; cat intravenous LD50: 5mg/kg; rabbit intravenous LDLo: 2400mg/kg
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 25.68
2. Molar volume (cm3/mol): 92.8
3. Isotonic specific volume (90.2K): 242.1
4. �Surface tension (dyne/cm): 46.2
5. Polarizability (10-24cm3): 10.18
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -3.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
p>
4. Number of rotatable chemical bonds: 3
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 63.3
p>
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 62.7
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Irritating.
2. Exist in flue-cured tobacco leaves and smoke.
Storage method
Store in a sealed and dry place away from light.
Synthesis method
1. There are synthesis methods and fermentation methods. 1. Synthetic method: Prepared by ring opening of pyrrolidone. Digest the quicklime into lime milk with distilled water, pump it into the hydrolysis reaction kettle, add pyrrolidone, raise the temperature to 125-130°C, keep the reaction pressure at 0.29MPa, and keep the reaction warm for more than 10-14 hours. After the reaction, the temperature was lowered to 30°C, the material was filtered, and washed with distilled water. Add ammonium bicarbonate to the filtrate until no calcium ions are detected, add activated carbon, incubate at 80°C for decolorization for 30 minutes, filter at 60°C, wash with distilled water, combine the washing liquid with the filtrate, concentrate under reduced pressure at 60°C until crystallization precipitates, add ethanol, After cooling, filtering and drying, the finished product is obtained with a yield of more than 85%. 2. Fermentation method uses Escherichia coli as the bacterial strain. The fermentation medium is bran hydrolyzate, corn steep liquor, peptone, magnesium sulfate and sodium chloride, etc. Soybean oil is used as the antifoaming agent, the dosage is about 0.1%, and the fermentation unit is about 100 enzyme units/ml of fermentation broth. In the refining process, the function of Escherichia coli decarboxylase is used to convert L-glutamic acid into γ-aminobutyric acid, which can be dissociated into cations in aqueous solution. Strongly acidic styrene-based cation exchange resin is used for ion exchange, and ammonia water is used. After elution, extraction, resin purification, concentration, crystallization and drying, the finished product is obtained.
2. Tobacco: FC, 51.
Purpose
1. Biochemical research. Organic Synthesis. Synthetic man-made fibers. 2. Pharmaceutical intermediates. 4-Aminobutyric acid has the effect of lowering blood lipids and is suitable for the treatment and prevention of various types of hepatic coma. It can treat poliomyelitis, cerebral hemorrhage, and can be used as an antidote for gas poisoning. Also used in biochemical research and organic synthesis 3.Clinically used for memory impairment, language impairment, partial paralysis caused by hepatic coma and cerebrovascular disorders, as well as sequelae of brain trauma and infantile spasm ,epilepsy. It is effective in treating convulsions and agitation in hepatic coma.

 微信扫一扫打赏
微信扫一扫打赏

