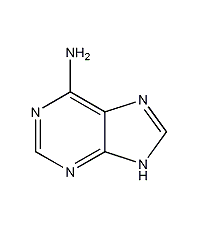
Structural formula
| Business number | 01HC |
|---|---|
| Molecular formula | C5H5N5 |
| Molecular weight | 135 |
| label |
1H-Purine-6-amine, 6-aminourea ring, 6-aminopurine, glandular urine ring, adenoidin, pancreatic acid, Vitamin B4, 6-Aminopurine, 6-Amino-1H-purine, 9H-Purin-6-ylamine, 3,6-Dihydro-6-iminopurine, 6-Aminopurine, Vitamin B4, Plant Growth Regulator, Heterocyclic compounds |
Numbering system
CAS number:73-24-5
MDL number:MFCD00041790
EINECS number:200-796-1
RTECS number:AU6125000
BRN number:5777
PubChem number:24891349
Physical property data
1. Properties: White fine powder crystals with a strong salty taste. The industrial product is slightly yellow crystal
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1) : Uncertain
4. Melting point (ºC): >360 (lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6 . Boiling point (ºC, 5.2 kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): 220
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25 ºC): Uncertain
12. Saturated vapor pressure (kPa, 60 ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical Temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
16. p>
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Dissolution Properties: Insoluble in cold water (0.5 g/L, 20 ºC), soluble in boiling water, acid and alkali, slightly soluble in ethanol, insoluble in ether and chloroform.
Toxicological data
Rat oral LD50: 745mg/kg
Ecological data
None, when adding materials, keep the internal temperature at 30-35°C. After the addition, heat the water bath to 37-40°C and stir for 1.5 hours. Pour the reaction solution into 25g of crushed ice, place, filter, wash with ice water until neutral, and filter with suction. Dried light yellow product.
4, 6-dihydroxypyrimidine [nitric acid, sulfuric acid] → [37-40℃, 1.5h] 4, 6-dihydroxy-5-nitropyrimidine
4, 6-dichloro-5 -Preparation of nitropyrimidine 120ml of phosphorus oxychloride, add 60g of 4,6-diamino-5-nitropyrimidine while stirring, heat to 50°C, add dimethylaniline dropwise, reflux for 1 hour after the dripping is completed, and complete the reaction Pour the liquid into 900g of crushed ice and leave it until all the ice cubes are dissolved. Filter and wash with ice water to obtain a light yellow product.
4, 6-dihydroxy-5-nitropyrimidine [phosphorus oxychloride, dimethylaniline] → 4, 6-dichloro-5-nitropyrimidine
4,6-diamino Preparation of -5-nitropyrimidine Dissolve 4,6-dichloro-5-nitropyrimidine in 300ml of ethanol, slowly reflux in a water bath and slowly add 400ml of ammonia water dropwise, keep the temperature at about 65°C during the dropwise addition, and continue to reflux after the addition 1.5h, cool and let stand overnight, then filter, wash with ice water and dry to obtain a brown product. 4, 6-dichloro-5-nitropyrimidine [ethanol, ammonia] → 4,6-diamino-5-nitropyrimidine
Preparation of 6-aminopurine phosphate formamide 200ml, add 4 under stirring , 26g of 6-diamino-5-nitropyrimidine and 30ml of formic acid, raise the temperature to 110°C, add 25g of insurance powder in batches, and control the internal temperature to 110-120°C, after adding, raise the temperature to 180-190°C, reaction 2.5 h, cooled overnight, filtered with suction, washed with ethanol and ice water until neutral, then recrystallized with 600 ml of distilled water, filtered with suction and dried to obtain light yellow 6-aminopurine. Add 250 ml of distilled water, add 0.0125 g of phosphoric acid, adjust the pH to 1-2, heat, decolorize the activated carbon, filter while hot, filter the filtrate after cooling, and dry to obtain the product.
4, 6-diamino-5-nitropyrimidine [methanol, formamide, sodium dithionite] → 6-aminopurine [phosphoric acid] → 6-aminopurine phosphate.
Purpose
1. Used for the production of adenosine, ATP, ADP, new anti-AIDS drugs, vitamin B4 and plant growth hormone 6-benzyladenine, etc.
2. Biochemical research and drug analysis can prevent and treat leukopenia caused by various causes. It is a component of nucleic acids and participates in the synthesis of RNA and DNA in organisms. When white blood cells are lacking, it can promote the proliferation of white blood cells. It is used to prevent and treat leukopenia and acute granulocytopenia. Also used for blood storage.
Note: Since this drug is a nucleic acid precursor, when used concurrently with tumor radiotherapy or chemotherapy, the possibility of promoting tumor development should be considered. During injection, it needs to be dissolved in 2 ml of disodium hydrogen phosphate buffer, injected slowly, and cannot be mixed with other drugs for injection.
3. It is an important intermediate in the preparation of adefovir dipivaloyloxymethyl ester.

 微信扫一扫打赏
微信扫一扫打赏

