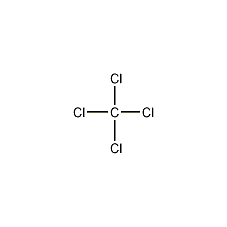
Structural formula
| Business number | 017B |
|---|---|
| Molecular formula | CCl4 |
| Molecular weight | 153.82 |
| label |
Tetrachloromethane, Perchloromethane, Tetrachloromethane, Perchloromethane, Benzimoform, Aliphatic halogenated derivatives |
Numbering system
CAS number:56-23-5
MDL number:MFCD00000785
EINECS number:203-453-4
RTECS number:203-453-4
BRN number:1098295
PubChem number:24857387
Physical property data
1. Properties: Colorless, transparent and volatile liquid with a special aromatic odor.
2. Boiling point (ºC, 101.3kPa): 76.72
3. Melting point (ºC): -22.92
4. Relative density (g/mL, 20/4ºC): 1.5942
5. Refractive index (n20ºC): 1.4604
6. Viscosity (mPa·s, 20ºC): 0.965
7. Heat of evaporation (KJ/mol, b.p.): 29.982
8. Heat of fusion (KJ/mol): 2.433
9. Heat of formation (KJ/mol, 25ºC, liquid): 135.5
10. Heat of combustion (KJ/mol, 25ºC, liquid): 258.24
11. Specific heat capacity (KJ/(kg·K), 20ºC): 0.866
12. Critical temperature (ºC): 283.15
13. Critical pressure (MPa): 4.56
14. Boiling point rise constant: 4.88
15 . Conductivity (S/m, 18ºC): 4×10-18
16. Volume expansion coefficient (K-1, 20ºC): 0.00127
17. Solubility: Miscible with most organic solvents such as water, alcohol, ether, petroleum ether, naphtha, glacial acetic acid, carbon disulfide, chlorinated hydrocarbons, etc.
18. Relative density (25℃, 4℃): 1.5846
19. Refractive index at room temperature (n25): 1.4573
20. Vapor pressure (kPa, 20ºC): 11.9102
21. Critical density (g·cm-3): 0.557
22. Critical Volume (cm3·mol-1): 276
23. Critical compression factor: 0.272
24. Eccentricity factor :0.193
25. Lennard-Jones parameter (A): 7.949
26. Lennard-Jones parameter (K): 633.6
27. Solubility parameter (J·cm-3)0.5:17.577
28. van der Waals area (cm2·mol-1): 7.280×109
29. van der Waals volume (cm3·mol– 1): 52.300
30.0831/201008311517435230.gif” alt=”” />
In addition to hydrochloric acid, the by-products include chloroform, tetrachloroethylene and hexachloroethane, all of which can be recycled and sold.
2 .Carbon disulfide method uses iron as a catalyst to react chlorine and carbon disulfide at 90 to 100°C. The reaction product is fractionated, neutralized, and rectified to obtain the finished product. This method has low investment and the product is easy to purify, but the cost is high and the equipment is seriously corroded.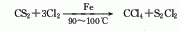
3. Methane oxychlorination method has high chlorine utilization rate. No pollution from hydrogen chloride and waste halogenated hydrocarbons.
4. The high-pressure chlorolysis method avoids the generation of tetrachlorethylene.
5. The methanol hydrochlorination method has good product quality and high economic benefits. In addition , both dichloromethane and chloroform production can co-produce tetrachloromethane.
6. Combine 100L industrial carbon tetrachloride, 15L 95% ethanol, 1.5g 42% sodium hydroxide and lead acetate Mix, stir, and heat and boil until the sulfide content test passes. Then add water and wash for more than three times. Discard the water layer and add concentrated sulfuric acid (10kg of sulfuric acid for every 100kg of carbon tetrachloride). Stir and pickle until the sulfuric acid test passes, and then wash with water. After three times, add 5% sodium hydroxide solution, stir well and let it stand, discard the water layer, and repeat alkali washing until the acidity is qualified. Add an appropriate amount of anhydrous calcium chloride and a small amount of anhydrous to the carbon tetrachloride that has passed the alkali washing. Sodium carbonate, let it stand fully, filter out the desiccant, distill it under normal pressure, and collect the 76-77.5°C fraction, which is the finished product.
Purpose
1. Mainly used as excellent solvents, dry cleaning agents, fire extinguishing agents, refrigerants, spice leaching agents and pesticides, etc.
2. It can be used to synthesize monomers of Freon, nylon 7, and nylon 9; it can also be used to prepare chloroform and drugs; it can be used as a lubricant in metal cutting.
3. Used as analytical reagents, such as standards and solvents for measuring chlorine. Also used as a cleaning agent, extraction solvent, and pesticide.
4. Used in organic synthesis as solvent, refrigerant, chlorinating agent for organic matter, leaching agent for spices, degreasing agent for fibers, etc. [17]

 微信扫一扫打赏
微信扫一扫打赏

