Background and overview[1]
2-Phenylethyl benzoic acid can be used as an intermediate in pharmaceutical synthesis, such as as an intermediate compound for the neurological drug amitriptyline, amitriptyline (also known as amitriptyline, elavir, and tricycline) , etc.), is an important clinical drug that can be used to treat a variety of mental diseases such as anxiety or motivating depression, panic disorder, emotional stress, mental disorder or gastrointestinal neurosis. It is currently clinically used One of the frequently used tricyclic antidepressants (its antidepressant effect is very similar to that of imipramine), its sedative effect is strong.
The mechanism of its treatment is to inhibit the reuptake of serotonin and norepinephrine, but the inhibition of serotonin reuptake is stronger, and the sedative and anticholinergic effects are also stronger, which can improve the mood of patients with depression. It can significantly improve symptoms such as slow thinking, slow behavior and loss of appetite; it can also relieve chronic pain by acting on central opioid receptors.
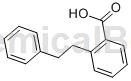
Preparation[1]
The preparation of 2-phenylethylbenzoic acid is as follows: add 100 mmol of the above formula ( I) compound, 100 mmol of the compound of formula (II) above, 8 mmol of catalyst bis(cyanomethyl)palladium dichloride, 10 mmol of nitrogen multidentate ligand, 180 mmol of oxidant bis(trifluoroacetate)iodobenzene and 200 mmol of organic base dimethylamino Pyridine, raise the temperature to 70°C, and stir the reaction at this temperature for 24 hours. After the reaction is completed, filter while hot, cool the filtrate to room temperature naturally, adjust the pH value to neutral, and then wash it 2-4 times with a sufficient amount of saturated sodium carbonate aqueous solution, combine the organic phases, cool to 2-6°C, and precipitate a solid. Filter with suction, wash thoroughly with absolute ethanol, and dry under vacuum to obtain the compound of formula (III) 2-phenylethylbenzoic acid in the form of white crystals with a yield of 97.2% and HPLC analysis showing that its purity is 98.7%. Melting point: 127.8-128.5℃.
1HNMR (CDCl3, 300MHz): δ3.04-3.14 (m, 4H, -CH2), 7.26-7.47 (m, 9H, -CH-), 12.12 (s, 1H, -OH).

Main reference materials
[1] CN201810099719.3 Synthesis method of neurodrug amitriptyline intermediate compound

 微信扫一扫打赏
微信扫一扫打赏

