Background and overview[1]
2,6-Dichlorophenyl isothiocyanate can be used as a pharmaceutical synthesis intermediate. If 2,6-dichlorophenyl isothiocyanate is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell. ; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
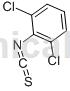
Preparation[1]
Method 1: Add 2.00mmol 2,6-dichlorophenyldithiocarboxylic acid triethylammonium salt into a 50mL eggplant-shaped flask, add 8.00mL acetonitrile and 4.0mmol triethylamine in sequence, and then stir at 0°C. During the process, 1.00 mmol of bistribromo1,3-bispyridinium propane was added in batches. After the addition was completed, it was kept at 0°C for 30 minutes, and then raised to room temperature to stir the reaction. During the reaction, TLC (thin layer chromatography, Developing agent: petroleum ether) to detect that the reaction was complete. After 16 hours of reaction, TLC detection showed that the reaction was complete, and then suction filtration was performed. The obtained filter cake was recovered (recovery method, please refer to the recovery of bistribromo1,3-dipyridinium base propane). Example), the obtained filtrate was concentrated, and the concentrated filtrate was subjected to column chromatography to obtain 2,6-dichlorophenyl isothiocyanate (0.129g, 29.5%) as a yellow solid, m.p.=40-41°C.

Method 2: Prepare the title compound 2,6-dichloroisothiocyanate using 2,6-dichloroaniline (2.0g, 12.34mmol), thiophosgene (1.41g, 12.34mmol) and N-ethyl The phenyl ester was eluted with a solution of diisopropylamine (7.42g, 57.51mmol) in DCM (20mL) to give the desired product, 2,6-dichlorophenylisothiocyanate, in a yield of 1.0g. 1HNMR (DMSO-d6): δ7.62 (d, J=7.8Hz, 2H), 7.39 (t, J=7.8Hz, 1H).
Main reference materials
[1] CN201410235515.X bistribromo1,3-dipyridinium propane and its preparation method, use method, recovery method and application
[2] PCTInt.Appl., 2012055995, 03May2012

 微信扫一扫打赏
微信扫一扫打赏

