Background and overview[1]
2,4,6-Trichlorobenzaldehyde can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
The preparation of 2,4,6-trichlorobenzaldehyde is as follows:
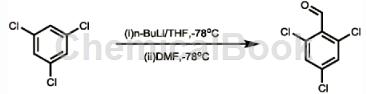
The specific steps are as follows: Under argon protection and minus 78 degrees Celsius, add 10.0g (55.1mmol) 1,3,5-trichlorobenzene and 100ml ultra-dry tetrahydrofuran into a 250ml single-neck flask. Then add 23ml (2.4M, 55.2mmol) n-butyllithium n-hexane solution dropwise, and stir for 30 minutes at minus 78 degrees Celsius. Then add 7.44ml (107.3mmol) ultra-dry DMF dropwise, and continue stirring at minus 78 degrees Celsius for 1.5 hours. Then the reaction system was slowly raised to room temperature, 200 ml (3M) hydrochloric acid solution was added to quench the reaction, and extracted three times with ethyl acetate, 25 ml each time. The organic layers were combined, washed with 25 ml of sodium bicarbonate solution, and dried over anhydrous sodium sulfate. After the solvent was evaporated to dryness, methylene chloride: petroleum ether = 1:3 (volume ratio) was used as the eluent, and purified by column chromatography to obtain 11.0g of the product 2,4,6-trichlorobenzaldehyde, with a yield of 95 %.
Apply[1]
2,4,6-Trichlorobenzaldehyde can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
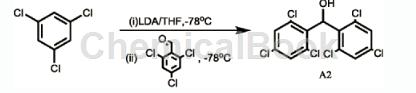
The specific steps are as follows: Under argon protection and minus 78 degrees Celsius, add 9.5g (52.4mmol) 1,3,5-trichlorobenzene and 100ml ultra-dry tetrahydrofuran into a 250ml single-neck flask. Then add 26.3 ml (2M, 52.6 mmol) lithium diisopropylamide solution in tetrahydrofuran/n-hexane dropwise, and stir at minus 78 degrees Celsius for 30 minutes. Then, 10.0 g (47.7 mmol) of compound 2,4,6-trichlorobenzaldehyde was added, and stirring was continued for 2 hours at minus 78 degrees Celsius. Then the reaction system was slowly raised to room temperature, 200 ml of saturated ammonium chloride solution was added to quench the reaction, and extracted three times with 25 ml of diethyl ether each time. The organic layers were combined, washed with 25 ml of sodium bicarbonate solution, and dried over anhydrous sodium sulfate. After the solvent was evaporated to dryness, dichloromethane: petroleum ether = 1:2 (volume ratio) was used as the eluent and purified by column chromatography to obtain 17.5g of product A2 with a yield of 94%.
Main reference materials
[1] (CN108191739) A room-temperature organic luminescent material based on benzyl radicals and an organic electroluminescent device prepared using the material

 微信扫一扫打赏
微信扫一扫打赏

