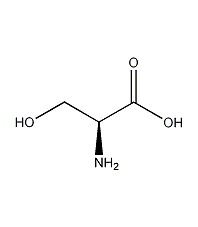
Structural formula
| Business number | 017N |
|---|---|
| Molecular formula | C3H7NO3 |
| Molecular weight | 105.09 |
| label |
(S)-2-Amino-3-hydroxypropionic acid, L-silk amino acid; L-β-hydroxyalanine, (S)-2-Amino-3-hydroxypropionic acid, L-β-hydroxyalanine, nutritional supplements, Intermediates |
Numbering system
CAS number:56-45-1
MDL number:MFCD00064224
EINECS number:200-274-3
RTECS number:VT8100000
BRN number:1721404
PubChem number:24899605
Physical property data
1. Properties: There are two types: levorotatory and racemic. The levorotatory is a white hexagonal prism crystal with a sweet taste; the racemate is a white monoclinic prism crystal. 2. Density (g/mL, 25/4℃): 1.415
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 223 ~228℃ (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): +15.1°(c=2,5mol /L hydrochloric acid)
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: L-body, soluble in Water, insoluble in anhydrous ethanol and ether; racemate. Slightly soluble in water, insoluble in colorless ethanol and ether.
Toxicological data
1. Mutagenicity: sister chromatids exchangeTEST system: human lymphocytes: 10mg/L
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 22.54
2. Molar volume (cm3/mol): 74.2
3. Isotonic specific volume (90.2K): 216.4
4, Surface tension (dyne/cm): 72.2
5, Polarizability (10-24cm3): 8.93
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 83.6
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 72.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable properties under normal temperature and pressure.
2. Exist in tobacco leaves and smoke.
Storage method
This product should be kept sealed and dry.
Synthesis method
1. DL-serine is synthesized from glycine, and L-serine is extracted from biological materials such as silk.
2.Hydrolysis method
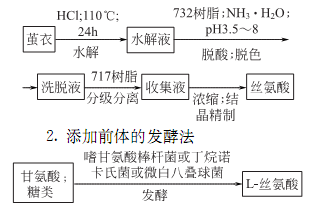
3.Chemical synthesis method
(1) Using hydroxyacetaldehyde Produced for raw materials.
(2) Use vinyl compounds as raw materials.
4. Enzymatic method
The intermediate DL-2 oxoxazolidine-4-carboxylic acid (DL-OOC) of chemically synthesized DL-serine is used as
5. Tobacco: BU, 22; FC, 21; Synthesis: It can be separated from protein (such as silk protein) hydrolyzate after removing tyrosine, glycine and alanine. Refined. It can also be obtained by amination and demethylation of a-bromo-β-methoxypropionic acid.
Raw material, serine can be produced using L-OOC hydrolase produced by Pseudomonas testosteroni or OOC racemase produced by Bacillus subtilis.
Purpose
1. Used as biochemical reagents and food additives.
2.Nutritional supplements, used as skin nutritional additives in cosmetics.
3. It can be used for biochemistry and nutritional research, and can also be used as a raw material for the synthesis of cycloserine.

 微信扫一扫打赏
微信扫一扫打赏

