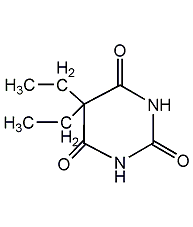
Structural formula
| Business number | 018M |
|---|---|
| Molecular formula | C8H12N2O3 |
| Molecular weight | 184.19 |
| label |
5,5-diethylbarbituric acid, 5,5-Diethyl-2,4,6(1H,3H,5H)-pyrimidinetrione, 5,5-Diethylbarbituric acid, Barbitone, Veronal |
Numbering system
CAS number:57-44-3
MDL number:MFCD00036212
EINECS number:200-331-2
RTECS number:CQ3500000
BRN number:163999
PubChem number:24891513
Physical property data
1. Characteristics: White Needle crystalline or crystalline powder. Odorless. Slightly bitter. Sublime in a vacuum.
2. Density ( g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,air=1): Undetermined
4. Melting point ( ºC): 188~192
5. Boiling point ( ºC,Normal pressure): Undetermined
6. Boiling point ( ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not OK
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturation vapor pressure (kPa,60ºC): Undetermined Molar volume (m3/mol):161.5
3. isotonic specific volume (90.2K):389.1
4. Surface Tension (dyne/cm):33.6
5. Polarizability(10-24cm3):17.47
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 5
6. Topological molecule polar surface area 75.3
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 247
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be kept sealed.
Synthesis method
diethyl malonate is obtained by ethylation, cyclization and acidification.
Purpose
Prepare buffer solution. Protein electrophoresis. Liver function tests. Hydrogen peroxide stabilizer. This product can be used as a hypnotic. Due to safety, effectiveness and drug resistance caused by repeated use, the use of barbiturates as hypnotic drugs has tended to decrease. Barbiturate tablets were released by the Department of Health1982year9One of the eliminated drugs announced in March. Barbiturates can be used as hydrogen peroxide stabilizers; used in the preparation of buffer solutions, protein electrophoresis, liver function testing, etc.
oma”>Diethyl malonate is obtained by ethylation, cyclization and acidification.
Purpose
Prepare buffer solution. Protein electrophoresis. Liver function tests. Hydrogen peroxide stabilizer. This product can be used as a hypnotic. Due to safety, effectiveness and drug resistance caused by repeated use, the use of barbiturates as hypnotic drugs has tended to decrease. Barbiturate tablets were released by the Department of Health1982year9One of the eliminated drugs announced in March. Barbiturates can be used as hydrogen peroxide stabilizers; used in the preparation of buffer solutions, protein electrophoresis, liver function testing, etc.

 微信扫一扫打赏
微信扫一扫打赏

