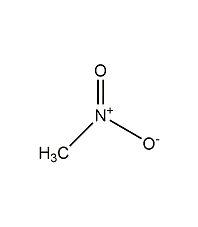
Structural formula
| Business number | 01JP |
|---|---|
| Molecular formula | CH3NO2 |
| Molecular weight | 61.04 |
| label |
Nitrocarbol, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:75-52-5
MDL number:MFCD00007400
EINECS number:200-876-6
RTECS number:PA9800000
BRN number:1698205
PubChem number:24845304
Physical property data
1. Properties: colorless oily liquid with fruity aroma. [1]
2. pH value: 6.12 (0.01mol/L aqueous solution) [2]
3. Melting point (℃): -29[3]
4. Boiling point (℃): 101.2[4]
5. Relative density (water=1): 1.14[5]
6. Relative vapor density (air=1): 2.11[6]
7. Saturated vapor pressure (kPa): 3.71 (20℃)[7]
8. Heat of combustion (kJ/mol): -708.1[8]
9. Critical temperature (℃): 315[9]
10. Critical pressure (MPa): 6.30 [10]
11. Octanol/water partition coefficient: -0.35[11]
12. Flash point ( ℃): 35 (CC) [12]
13. Ignition temperature (℃): 418[13]
14. Explosion upper limit (%): 63.0[14]
15. Explosion lower limit (%): 7.1[15]
16. Solubility: Slightly soluble in water, soluble in ethanol, ether, and dimethylformamide. [16]
Toxicological data
1. Acute toxicity[17]
LD50: 940mg /kg (orally in rats); 1440mg/kg (orally in mice)
2. Irritation No information available
3 .Carcinogenicity [18] IARC Carcinogenicity Comment: G2B, suspected human carcinogen.
Ecological data
1. Ecotoxicity[19]
LC50: 460mg/L (48h) (zebrafish, static); <278mg/L (96h) (fathead minnow, static)
2. Biodegradability [20] Sealed bottle test, initial concentration 2ppm, after 4 weeks Degradation is 4%, initial concentration is 10ppm, degradation is 5% after 4 weeks.
3. Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 12.70
2. Molar volume (cm3/mol): 57.8
3. Isotonic specific volume (90.2K ): 130.4
4. Surface tension (dyne/cm): 25.9
5. Polarizability (10-24cm3): 5.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 27.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertainty principle�Make the solution pH=8~9, control the temperature below 20℃, then add 42% sodium nitrite solution, mix evenly, slowly heat in a reactor with a reflux device until carbon dioxide gas is generated, stop heating, and let The reaction proceeds automatically:
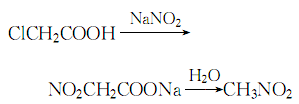
The entire reaction process , control the reaction temperature between 80 and 110°C. Due to the exothermic reaction, when the temperature exceeds 85°C, the heating should be stopped. At 90°C, nitromethane and water are evaporated at the same time. Collect the distillate, let it stand for layering, discard the water layer, dry the oil layer with anhydrous calcium chloride, distill under normal pressure, and collect the 100-101°C fraction. , which is the finished product.
Purpose
1. Nitromethane has great polarity and is miscible with many organic compounds. It is a good solvent and can be used as nitrocellulose, cellulose acetate, vinyl resin, polyacrylate paint and beeswax. etc. solvents.
2. It can be used to prepare explosives, rockets, fuels, medicines, dyes, pesticides, fungicides, stabilizers, surfactants and gasoline additives, etc. It is mainly used as a polar solvent in adhesives. It is miscible with many organic compounds and can dissolve cellulose derivatives, resins, dyes, greases, etc., especially for nitrocellulose, cellulose acetate, polyacrylonitrile, polyacrylonitrile, etc. Ester, wax products, etc. have good solubility.
3. Used as aerosol propellant, rocket fuel and the manufacture of explosives, dyes, etc.
4. Used as solvent, rocket fuel, gasoline additive and in organic synthesis. [27]

 微信扫一扫打赏
微信扫一扫打赏

