Background and overview[1]
4-morpholinopropiophenone can be used as a pharmaceutical synthesis intermediate. If 4-morpholinopropiophenone is inhaled, move the patient to fresh air; if skin contact occurs, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if contact with eyes , you should separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
4-morpholinopropiophenone is prepared as follows:

The specific steps are as follows: Add a mixture of 1-(4-fluorophenyl)ethanone (5g, 36.20mmol), morpholine (6.31g, 72.39mmol) and K2CO3 (10g, 72.39mmol) in DMF at 100 Stir overnight at ℃. The reaction mixture was cooled to ambient temperature and evaporated to remove the solvent. The residue was purified by silica gel column chromatography (4 × 16 cm, eluting with 10% ethyl acetate/petroleum ether) to give the title compound 4-morpholinopropiophenone (2.63 g, 35.4%) as a yellow solid. 1HNMR (300MHz, CDCl3) 68.40 (d, J=8.4Hz, 2H), 8.70 (d, J=8.4Hz, 2H), 3.88-3.85 (m, 4H), 3.33-3.29 (m, 4H), 2.53 ( s, 3H); LC-MS>95% (purity), 206 (MH)+.
Application
4-morpholinopropiophenone can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
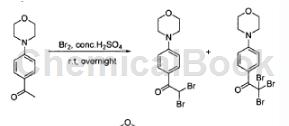
The specific steps are as follows: Cool the solution of 4-morpholinopropiophenone (5g, 24.36mmol) in concentrated sulfuric acid (30mL) to 0°C. Bromine (1.28 mL, 25.09 mmol) was slowly added to the solution. The resulting mixture was gradually warmed to ambient temperature and stirred for a further 6 hours. Pour the reaction mixture into ice water. The precipitate formed was filtered, washed with water and air dried to give the product as a green solid. LC-MS 363.9 (MH)+ (78%) and 442.8 (MH)+ (22%).
Main reference materials
[1](WO2010028193)COMPOUNDSINCLUDINGPIMELICACIDDERIVATIVESASHDACINHIBITORS

 微信扫一扫打赏
微信扫一扫打赏

