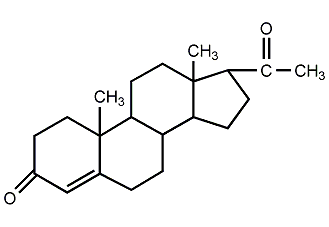
Structural formula
| Business number | 0190 |
|---|---|
| Molecular formula | C21H30O2 |
| Molecular weight | 314.47 |
| label |
progesterone, 4-Pregnene-3,20-dione |
Numbering system
CAS number:57-83-0
MDL number:MFCD00003658
EINECS number:200-350-6
RTECS number:TW0175000
BRN number:1915950
PubChem number:24278618
Physical property data
1. Appearance: white crystal or powder . There are two kinds of crystals that transform into each other, αThe type is columnar,βThe type is needle-shaped and has the same physiological activity.
2. Density ( g/mL,25/4℃) : Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point ( ºC): (αtype)127-131℃, (βtype)121℃
5. Boiling point ( ºC,Normal pressure): Undetermined
6. Boiling point ( ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not OK
11. Vapor pressure (kPa,25ºC<SPAN style="FONT-SIZE: 9pt; FONT-FAMILY: Arial; mso-ascii-font-family: Arial; mso-font-kerning: 0p288.9
3. isotonic specific volume (90.2K):731.9
4. Surface Tension (dyne/cm):41.1
5. Polarizability(10-24cm3): 36.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 15
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 23
8. Surface charge: 0
9. Complexity: 589
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 6
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a cool, dry place away from light.
Synthesis method
Obtained from the oxidation of pregnenolone. Add dry toluene into a dry reaction pot, add cyclohexanone and pregnenolone, stir to dissolve, steam toluene to dehydrate, quickly add aluminum isopropoxide, and place in 115℃ oxidation reaction2h, cool to80℃ or so , add while stirring5%Dilute sulfuric acid, let it stand and separate the layers, separate the water layer, wash the toluene layer with water until it is neutral, and then perform steam distillation. Steam off toluene and cyclohexanone. Cool, filter, and stir the filter residue into a slurry with petroleum ether, filter, wash with petroleum ether, and dry to obtain crude progesterone. Dissolve the crude product in ethanol, decolorize with activated carbon, and recrystallize the finished product, yield80%.
Purpose
hormone drugs. It mainly promotes and maintains uterine changes in early pregnancy and is used for habitual miscarriage, irregular menstruation, etc.
mso-bidi-language: AR-SA; mso-bidi-font-family: Arial; mso-hansi-font-family: Arial”>℃ oxidation reaction2h, cool to80℃, add under stirring5%rare sulfuric acid, let it stand for layering, separate the water layer, wash the toluene layer with water until neutral, then perform steam distillation to evaporate toluene and cyclohexanone. Cool, filter, and stir the filter residue into a slurry with petroleum ether, filter, wash with petroleum ether, and dry to obtain crude progesterone. Dissolve the crude product in ethanol, decolorize with activated carbon, and recrystallize the finished product, yield80%.
Purpose
hormone drugs. It mainly promotes and maintains uterine changes in early pregnancy and is used for habitual miscarriage, irregular menstruation, etc.

 微信扫一扫打赏
微信扫一扫打赏

