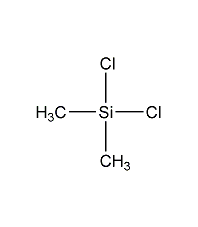
Structural formula
| Business number | 01K3 |
|---|---|
| Molecular formula | C2H6Cl2Si |
| Molecular weight | 129.06 |
| label |
Dichlorodimethylsilane, dimethyl silicon dichloride, Dimethyldichlorosilane, dimethyldichlorosilane, Dimethyldichlorosilane, DMDCS, Dow Corning® product Z-1219, Elemental organic compounds |
Numbering system
CAS number:75-78-5
MDL number:MFCD00000491
EINECS number:200-901-0
RTECS number:VV3150000
BRN number:605287
PubChem number:24867582
Physical property data
1. Characteristics: colorless fuming liquid with pungent odor. [1]
2. Melting point (℃): <-70[2]
3. Boiling point (℃) : 70.5[3]
4. Relative density (water = 1): 1.07[4]
5. Relative Vapor density (air=1): 4.45[5]
6. Saturated vapor pressure (kPa): 14.5 (20℃)[6]
7. Critical pressure (MPa): 3.49[7]
8. Octanol/water partition coefficient: 2.24[8]
9. Flash point (℃): -9 (CC); -16 (OC) [9]
10. Ignition Temperature (℃): 398.9[10]
11. Explosion limit (%): 9.5[11]
12 .Lower explosion limit (%): 3.4[12]
13. Solubility: soluble in benzene and ether. [13]
Toxicological data
1. Skin/eye irritation
Standard Draize test: rabbit, skin contact: 20mg/24H; severity of reaction: moderate.
Standard Draize test: Rabbit, eye contact: 5 mg/24H; Severity of reaction: Severe.
2. Acute toxicity:
Rat oral LD50: 5660μL/kg; mouse inhalation LC50: 930ppm/4H; rat intraperitoneal LDLo: 10mg/kg; small Rat inhalation LC50: 300mg/m3/2H;
3. Acute toxicity[14] LC50: 4910mg /m3(rat inhalation, 4h)
4. Irritation [15]
Rabbit transdermal: 20mg (24h), moderate irritation.
Rabbit eye: 5mg (24h), severe irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 29.73
2. Molar volume (cm3/mol): 120
3. Isotonic specific volume (90.2K ): 247.7
4. Surface tension (dyne/cm): 18.1
5. Polarizability (10-24cm3): 11.78 p>
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 30.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product is highly toxic. Irritating to respiratory tract and skin. After long-term exposure, symptoms such as nasal mucosa atrophy or bronchitis may occur. In addition, there are symptoms such as headache, drowsiness, weakness, and chest pain. Hydrogen chloride is released when it comes in contact with water, which is also irritating and corrosive, so special attention should be paid during operation. Rinse with plenty of water after contact with skin and eyes, equipment should be sealed, and operators should wear protective gear.
2. Stability[16] Stable
3. Incompatible substances[17] Strong oxidants, acids, alcohols, amines, strong bases
4. Conditions to avoid contact[18] Humid air, heat
5. Polymerization hazard[19] No polymerization
6. Decomposition products [20] Silicon oxide, hydrogen chloride
Storage method
Storage Precautions[21] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, alkalis, alcohols, etc. and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
(1) The direct method is now used in industry. During production, copper or copper salt is used as a catalyst to directly react methyl chloride with silicon powder at about 300°C. This is a complex heterogeneous reaction. The reaction mixture mainly contains dimethyldichlorosilane (75%), Monomethyltrichlorosilane (20-30%), trimethylmonochlorosilane (5%) and methyldichlorosilane; the boiling points of these components are 70°C, 66.4°C, 57.9°C and 41°C respectively , using an emulsification tower for separation. If necessary, its composition can be adjusted by changing operating conditions.
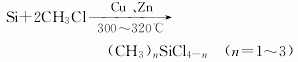
(2) Grignard reagent method After long-term efforts, F.S. Kipping and his collaborators synthesized a large number of organosilicon compounds using the Grignard reagent method. Its characteristics are: the product has fewer components, can be used to prepare mixed hydrocarbyl silanes, can produce metal salts as a by-product, and can connect the same organic groups such as alkyl groups, alkenyl groups, aryl groups, etc. to silicon atoms to form monomers. The disadvantages are: it requires the use of flammable ether or other solvents, which not only consumes a lot of money, but is also flammable and explosive, making production unsafe; the process steps are many and complex; the raw materials silicon tetrachloride and Grignard reagent are expensive and costly, and are not suitable For large-scale industrial production.
(3) Condensation method (substitution method) A method in which chlorosilane (mainly hydrogen-containing chlorosilane) reacts with hydrocarbons or halogenated hydrocarbons, and is condensed to generate organochlorosilane under high temperature or the action of a catalyst. , also known as heat shrinkage method. Think of the condensation process as a process in which hydrogen or chlorine on the silane is replaced by a hydrocarbon group. The condensation method can also be called a substitution method.
(4) Disproportionation (redistribution) method refers to the method of using a certain catalyst to redistribute the substituents in silane to generate new organohalosilanes, so it is also called the redistribution method. Its outstanding advantage is that it can solve the imbalance problem in monomer production, realize comprehensive utilization and reduce production costs.
Purpose
1. Used in the manufacture of dimethyl silicone oil, silicone rubber and silicone resin.
2. Used as an intermediate in the manufacture of silicone resin. [22]

 微信扫一扫打赏
微信扫一扫打赏

