Background and overview[1]
1-(2-Fluoro-3-hydroxy-6-nitrophenyl)propan-2-one can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
The preparation of 1-(2-fluoro-3-hydroxy-6-nitrophenyl)propan-2-one is as follows:
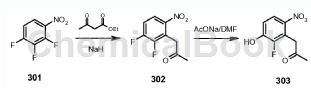
Step 1): 1-(2,3-difluoro-6-nitrophenyl)propan-2-one (compound 302)
To a suspension of sodium hydride (5.42g, 226mmol) in THF (100mL) was added ethyl acetoacetate (29.4g, 226mmol) while maintaining the reaction temperature below 15°C. Stir the mixture for 15 minutes. After completion of the addition, a solution of compound 301 (20.0 g, 113 mmol) in THF (150 mL) was added to the mixture while maintaining the reaction temperature below 50 °C. The mixture was then stirred at room temperature for 24 hours. The solvent was removed in vacuo and the residue was partitioned between ethyl acetate and 2N aqueous hydrochloric acid. The organic layer was washed with water, brine, dried over MgSO4 and concentrated. Concentrated hydrochloric acid (400 mL) and acetic acid (300 mL) were then added to the residue, and the mixture was refluxed for a further 12 hours. After cooling, the mixture was concentrated and the residue was partitioned between 5% sodium bicarbonate and ethyl acetate. The organic layer was washed with water, brine, dried over MgSO4 and concentrated. The residue was purified by silica gel column chromatography (EtOAc/petroleum ether = 1/2) to obtain compound 302 (14.5 g, 60%) as a brown oil: LCMS: 216 [M+1]+.
Step 2): 1-(2-fluoro-3-hydroxy-6-nitrophenyl)propan-2-one (compound 303)
A mixture of 302 (4.30g, 0.02mol), AcONa (1.72g, 0.021mol) and DMF (40mL) was stirred at 100°C for 12 hours. The mixture was then filtered, the solvent was removed under reduced pressure, and the residue was extracted with ethyl acetate (100 mL). The organic layer was washed with water, brine, dried over MgSO4 and concentrated. The residue was purified by silica gel column chromatography (EtOAc/petroleum ether = 1/1) to obtain compound 1-(2-fluoro-3-hydroxy-6-nitrophenyl)propan-2-one 303 (2.3 g, 54% ), is a light yellow solid: LCMS: 214[M+1]+; 1HNMR (DMSO-J6): δ2.30 (s, 3H), 4.26 (s, 2H), 7.67 (m, 1H), 8.05 (m ,1H).
Main reference materials
[1](WO2009036055)VEGFRINHIBITORSCONTAININGAZINCBINDINGMOIETY

 微信扫一扫打赏
微信扫一扫打赏

