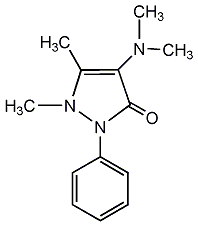
Structural formula
| Business number | 0198 |
|---|---|
| Molecular formula | C13H17N3O |
| Molecular weight | 231.3 |
| label |
Analytical identification reagents |
Numbering system
CAS number:58-15-1
MDL number:MFCD00003142
EINECS number:200-365-8
RTECS number:CD2625000
BRN number:None
PubChem number:24894178
Physical property data
1. Properties: White leaf-like crystals or crystalline powder. Odorless. Slightly bitter taste. Stable in air, but will decompose when exposed to sunlight.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 107-109
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: In the presence of moisture, it easily reacts with oxidants Have a chemical reaction. 1g product is dissolved in 1.5ml ethanol, 12ml benzene, 1ml chloroform, 13ml ether and 18ml water. Its solubility in water increases with the addition of sodium benzoate, and the aqueous solution is weakly alkaline to litmus.
Toxicological data
Low toxicity, LD50 (rat, oral) 1700 mg/kg. Irritating.
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 68.33
2. Molar volume (cm3/mol): 196.0
3. Isotonic specific volume (90.2K): 518.8
4. Surface tension (dyne/cm): 49.0
5. Polarizability (10-24cm3): 27.08
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological moleculesPolar surface area 26.8
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 343
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be kept sealed, dry and protected from light.
Synthesis method
1. Obtained from catalytic hydrogenation (alkylation) of aminoantipyrine. First add water to the dissolving tank, heat it to 50-60°C, add aminoantipyrine under stirring, and pour it into the hydrogenation tank after it is completely dissolved. Wash the dissolving tank with water, mix it with the nickel catalyst and then add it to the hydrogenation tank. Evacuate the hydrogenation tank, then close the vacuum valve, pass in hydrogen, start stirring, and stop passing hydrogen when the pressure rises to 0.245MPa. Add formaldehyde and continue to pass hydrogen gas. The speed of adding formaldehyde is based on the standard of absorbing 1.8-2 cubic meters of hydrogen for every 5L of formaldehyde added. The reaction temperature is controlled at 60-85°C. After the formaldehyde is added, the reaction continues for 10 minutes and the test end point is reached. After passing the test, press filtrate, cool the filtrate to below 25°C, precipitate crystals, and filter out to obtain crude aminopyrine. Warm the crude aminopyrine, ethanol, and activated carbon to 75-80°C, stir and decolorize for 1 hour, and press filter. The filtrate is cooled to 10°C, crystals precipitate, filtered, washed with ethanol, and dried by airflow to obtain aminopyrine.
2.Crude aminopyrine is obtained by catalytic hydrogenation of aminoantipyrine. Raise the crude aminopyrine, ethanol and activated carbon to a certain temperature, stir and decolorize, press filter, cool and crystallize, wash and dry.
Purpose
1. Determination of antimony, bismuth, bromine, cadmium, chlorine, cobalt, copper, cyanide, gold, iron, iodic acid, lead, nickel, nitric acid, nitrite, osmium, nitrogen oxide, palladium, platinum , silver and zinc. Determination of copper, iron and silica.
2. Antipyretic and analgesic drugs, used for fever, headache, joint pain, neuralgia, dysmenorrhea and active rheumatism. Aminopyrine is a pyrazolone non-steroidal anti-inflammatory drug with strong antipyretic, analgesic, anti-inflammatory and anti-rheumatic effects. However, due to serious adverse reactions, such as agranulocytosis, aplastic anemia, etc. Therefore, its clinical application has been reduced day by day and is gradually being eliminated. Aminopyrine can also be used as an analytical reagent.
3.Aminopyrine can be used as an analytical reagent to determine antimony, bismuth, bromine, cadmium, chlorine, and cobalt , ketones, cyanides, gold, ferric iodic acid, lead, nickel, nitric acid, nitrites, osmium, nitrogen oxides, palladium, platinum, silver and zinc. It is also an antipyretic and analgesic drug, used for fever, headache, joint pain, neuralgia, dysmenorrhea and active rheumatism. Aminopyrine is a pyrazolone non-steroidal anti-inflammatory drug with strong antipyretic, analgesic, anti-inflammatory and anti-rheumatic effects. But it can easily cause agranulocytosis, aplastic anemia, etc. Its clinical application has been increasingly reduced.

 微信扫一扫打赏
微信扫一扫打赏

