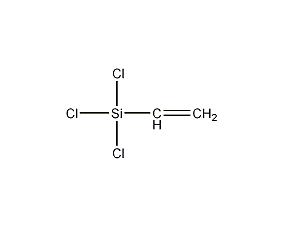
Structural formula
| Business number | 01KE |
|---|---|
| Molecular formula | C2H3Cl3Si |
| Molecular weight | 161.49 |
| label |
vinyltrichlorosilane, Ethenyl trichlorosilane, Coupling agent |
Numbering system
CAS number:75-94-5
MDL number:MFCD00000482
EINECS number:200-917-8
RTECS number:VV6125000
BRN number:1743440
PubChem number:24846680
Physical property data
1. Properties: colorless to light yellow liquid with suffocating odor. [1]
2. Melting point (℃): -95[2]
3. Boiling point (℃): 90.6[3]
4. Relative density (water=1): 1.27 (25℃)[4]
5. Relative vapor density (air = 1): 5.6[5]
6. Saturated vapor pressure (kPa): 8.78 (25℃)[6]
7. Heat of combustion (kJ/mol): -1614.9[7]
8. Critical pressure (MPa): 3.49[8]
9. Octanol/water partition coefficient: 2.36[9]
10. Flash point (℃): 11 (CC); -9[10]
11. Ignition temperature (℃): 262.78[11]
12. Explosion upper limit (%): No data yet
13. Explosion lower limit (%): 3[12]
14. Solubility: Soluble in most organic solvents. [13]
Toxicological data
1. Skin/eye irritation
Open irritation test: rabbit, skin contact: 625mg, severity of reaction: mild.
2. Acute toxicity: Rat oral LD50: 1280mg/kg; rat inhalation LCLo: 500ppm/4H; rabbit skin contact LD50: 680μL/kg.
3. Acute toxicity [14]
LD50: 1280mg/kg (rat oral); 0.68ml ( 863.6mg)/kg (rabbit transdermal)
4. Irritation[15] Rabbit transdermal: 625mg, severe irritation (open Stimulation test)
Ecological data
It is extremely destructive to the ozone layer of the atmosphere.
Molecular structure data
1. Molar refractive index: 34.30
2. Molar volume (cm3/mol): 126.5
3. Isotonic specific volume (90.2K ): 275.2
4. Surface tension (dyne/cm): 22.3
5. Polarizability (10-24cm3): 13.59 p>
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 54.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters : 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond unitsQuantity: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible substances[17] Strong oxidants, strong acids, strong alkali, water
3. Conditions to avoid contact[18] Humid air
4. Polymerization hazard[19] Polymerization
5. Decomposition products[20] Hydrogen chloride
Storage method
Storage Precautions[21] Usually products contain polymerization inhibitors. Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, alkalis, etc. and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. The addition reaction of silicon hydride to substituted alkenes and acetylene is the most important laboratory preparation method and industrial production method. Vinyl trichlorosilane can be produced by adding trichlorosilane to the reactor, heating and introducing acetylene under stirring, and performing a gas-liquid phase addition reaction, or heating and vaporizing trichlorosilane and mixing it with acetylene for a gas-phase addition reaction. 
2. There are three types of synthesis of vinyltrichlorosilane Method: Direct reaction of vinyl chloride and silicon powder; thermal condensation reaction of vinyl chloride and trichlorosilane; hydrosilylation reaction of acetylene and trichlorosilane. Among them, the use of thermal condensation reaction to generate vinyl trichlorosilane has become large-scale. The reaction formula is as follows:![]()
With trichlorosilane and Acetylene is used as raw material to synthesize vinyltrichlorosilane, which is an important laboratory and industrial preparation method:
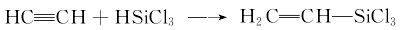
Add trichlorosilane into the reactor, introduce acetylene under stirring, and perform a gas phase addition reaction; or heat and vaporize trichlorosilane and perform a gas phase addition reaction with acetylene, both of which can be made. Obtain vinyl trichlorosilane.
Purpose
1. Used for the surface treatment of glass fiber and the treatment of reinforced plastic laminates. It can undergo a condensation reaction with the silanol on the glass surface, form a covalent bond with the glass, and improve the relationship between the glass fiber and unsaturated polyester, epoxy Adhesion, heat resistance, and water resistance of resin, acrylic resin, etc. It is also used as a coupling agent for rubber filler silica and as a modifier for chlorine-containing resins and as a raw material for the synthesis of various silicone products.
2. Used in organic synthesis, as an intermediate in silicone manufacturing, a coupling agent for adhesives, and a chemical reagent. [22]

 微信扫一扫打赏
微信扫一扫打赏

