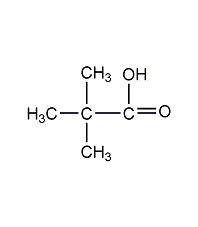
Structural formula
| Business number | 01KJ |
|---|---|
| Molecular formula | C5H10O2 |
| Molecular weight | 102 |
| label |
2,2-Dimethylpropionic acid, pivalic acid, pivalic acid, 2,2-Dimethylpropanoic acid, Pivalic acid, Neopentanoic acid, Aliphatic carboxylic acids and their derivatives, acidic solvent |
Numbering system
CAS number:75-98-9
MDL number:MFCD00004194
EINECS number:200-922-5
RTECS number:TO7700000
BRN number:969480
PubChem number:24887585
Physical property data
1. Properties: colorless needle crystals
2. Relative density (g/mL, 50/4℃): 0.905
3. Relative density (25℃, 4 ℃): 0.90550
4. Melting point (ºC): 35
5. Boiling point (ºC, normal pressure): 164, 70ºC (1866pa)
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index (36.5ºC): 1.3931
8. Flash point (ºC) : 63
9. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2891.6
10. Gas phase standard claimed heat (enthalpy) )(kJ·mol-1): -505.0
11. Liquid phase standard heat of combustion (enthalpy) (kJ·mol-1): -2832.1
12. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -564.4
13. Heat of formation (KJ/ mol, 25ºC): -564.80
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Dissociation constant (25ºC, water): 9.76×10-5
17. Explosion upper limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Easily soluble in ethanol, ether, and water. 1g of tert-pentanoic acid can be dissolved in 40mL of water.
Toxicological data
Animal tests show low toxicity.
Ecological data
None
Molecular structure data
1. Molar refractive index: 26.74
2. Molar volume (cm3/mol): 105.7
3. Isotonic specific volume (90.2K ): 247.9
4. Surface tension (dyne/cm): 30.2
5. Polarizability (10-24cm3): 10.60
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.5
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 37.3
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 78.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the stereocenter of chemical bonds Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Corrosive to metals. Due to the steric hindrance, the carboxyl group is protected, so the esterification speed of tert-pentanoic acid is slow, and the generated ester is also difficult to hydrolyze.
2. This product is corrosive. Rat oral administration LD50900mg/kg, rabbit skin application LD501900mg/kg.
3. It is corrosive and may cause combustion; the same precautions as for acetic acid should be adopted.
4. Found in flue-cured tobacco leaves.
Storage method
1. This product should be sealed and stored in a cool place.
2. Packed in wooden barrels lined with plastic bags. Pay attention to moisture and sun protection, and store in a cool, dry and ventilated place. Store and transport according to general chemical regulations.
3. Storage temperature 4ºC
Synthesis method
1. Isobutanol and formic acid react under the action of concentrated sulfuric acid to obtain tert-valeric acid.
![]()
2. Add sulfuric acid to the autoclave , replace the air in the kettle with carbon monoxide, and then fill it with carbon monoxide to bring the pressure to about 5MPa. Add the mixture of isobutylene and chloroform using a metering pump. Stir at room temperature for half an hour at about 5MPa. After the pressure is released, pour the material into ice water and stir at 5-15°C for 15 minutes. Separate the chloroform layer, dry with anhydrous sodium sulfate, and distill. Collect the 65-70°C (2.67kPa) fraction to obtain trimethylacetic acid with a purity of 97%. The yield is 74%.
![]()
3. Tert-butyl alcohol and formic acid (98%) prepared by reaction in the presence of concentrated sulfuric acid.
![]()
4. Tobacco: FC, 40 . Prepared by the reaction of tert-butyl magnesium chloride and carbon monoxide.
5. It can be prepared by the reaction of bromoform and pinanolone or the reaction of tert-butyl chloride, magnesium and carbon dioxide. It can also be obtained by the reaction of tert-butyl chloride, lithium and 2-ethoxyethoxymagnesium.
6. Preparation method:
In a reaction bottle equipped with a stirrer, thermometer, reflux condenser and dropping funnel, add 700mL of water and 80g of sodium hydroxide (2mol) , after dissolving, cool to 0°C in an ice-salt bath. Slowly add 120g (0.75mol) of bromine dropwise while stirring, keep the reaction temperature below 10°C, and complete the addition in about 20 minutes. Cool to 0℃, slowly add 25g (0.25mol) of metanone (2), and control the reaction solution not to exceed 10℃. After the addition was completed, slowly raised to room temperature and stirred for 3 hours. Steam distillation to remove the bromoform produced. After distillation, cool and slowly add sulfuric acid to make it acidic. Then carry out steam distillation, and when the upper layer is 16g of oily methylpropionic acid (1), it solidifies after cooling, mp34~35℃. The yield is 55%. [1]
Purpose
1. Used as raw material for the production of olefin polymerization initiator TBPP, as well as raw materials for the production of polyvinyl chloride stabilizers and spices. Used in the manufacture of pharmaceuticals, adhesives, initiators, fragrances and stabilizers for polyvinyl chloride.
2.Acid-catalyzed asymmetric reduction When studying the application of Raney nickel in asymmetric hydrogenation reactions, it was found that using pivalic acid as a catalyst can obtain higher optical purity product. The reduction reaction of 2-octanone to 2-octanol under the action of tartaric acid-sodium bromide modified Raney nickel. For example, using a THF/carboxylic acid mixture as the reaction medium can improve the optical purity, and using pivalic acid can reach the maximum value. When various methyl ketones are reduced by catalytic hydrogenation using modified Raney nickel in the presence of pivalic acid, the corresponding (S)-RCH(OH)Me compounds with high optical purity can be obtained.
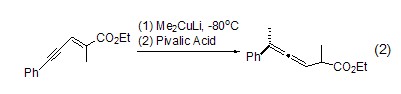
Acid-catalyzed rearrangement Using propionic acid as a catalyst, o-methoxyphenol and triethyl n-acrylate are refluxed in benzene to obtain the Claisen rearrangement ring-closure product. The yield is only 50% because the catalyst is esterified. Using pivalic acid, which is more sterically hindered, as a catalyst, a very high yield can be obtained (Formula 1)[1,2].

Enol region Selective protonationIn the study of regioselective protonation of allenyl enol, pivalic acid showed strong selectivity, and its intermediate alkene at –80 oC Alcohols can be specifically protonated at the C-2 position when quenched by pivalic acid (formula 2)[3].
Obtain tert-butyl through oxidative decarboxylation The decarboxylation reaction of carboxylic acids catalyzed by silver peroxodisulfate can produce alkyl radicals, which can be used to convert alkyl radicals into aromatic bases. base. In this process, pivalic acid can serve as a tert-butyl donor, and 2-tert-butylquinoline is regioselectively synthesized through this method (Formula 3)[4].
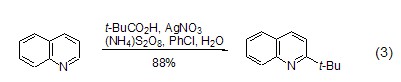
Preparation of 2-tert-butylpyrimidine 2-tert-butylpyrimidine is the precursor of the insecticide 2-tert-butylpyrimidinyl phosphorothioate. 2-tertiary butylpyrimidine can be synthesized from the reaction of butyldiamine and pivalic acid [5].
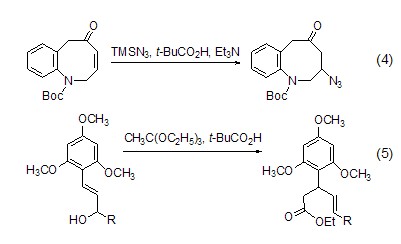
Introduce other nucleophilic groups at the γ-position of enone or enol For example, use TBSN3, t-BuCO2H and Et3N react with enone to introduce an azide group at the γ position (Formula 4, Formula 5)[6,7].
strong> Silver peroxydisulfate catalyzes the decarboxylation reaction of carboxylic acids to produce alkyl radicals, which can be used for the alkylation of aromatic bases. In this process, pivalic acid can serve as a tert-butyl donor, and 2-tert-butylquinoline is regioselectively synthesized through this method (Formula 3)[4].
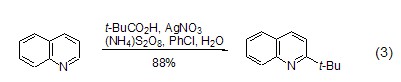
Preparation of 2-tert-butylpyrimidine 2-tert-butylpyrimidine is the precursor of the insecticide 2-tert-butylpyrimidinyl phosphorothioate. 2-tertiary butylpyrimidine can be synthesized from the reaction of butyldiamine and pivalic acid [5].
Introduce other nucleophilic groups at the γ-position of enone or enol For example, use TBSN3, t-BuCO2H and Et3N react with enone to introduce an azide group at the γ position (Formula 4, Formula 5)[6,7].


 微信扫一扫打赏
微信扫一扫打赏

