Background and overview[1][2]
5-Chloro-3-benzofuranone is a ketone derivative and can be used as a pharmaceutical synthesis intermediate.
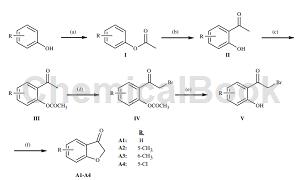
Preparation method[1]
5-Chloro-3-benzofuranone was prepared as follows: phenol derivative (0.5 mol) (R: 5-chloro) and acetic anhydride (0.6 mol) were refluxed with pyridine for 30 minutes, and then the reaction mixture was cooled with Treat the water, filter the precipitate, and extract the solid material with an appropriate solvent. Place the obtained phenyl acetate derivative (I) (0.4 mol) in an oil bath, react with AlCl3 (0.48 mol) in the flask, pour the viscous mixture after leaving the oil bath, and put it into ice water to dry. Dry to obtain 20-hydroxyacetophenone derivative (II). Compound II (0.3 mol) was stirred with acetic acid and anhydride (0.45 mol) was added at 50-100°C for 3-4 hours. In order to prevent the reactivity of the hydroxyl group, it was acetylated. Obtain 20-acetoxyacetophenone derivative (III) (0.25mol); brominate the compound with Br2 (0.3mol) in diethyl ether or acetic acid to obtain 20-acetoxy-2-bromoacetophenone derivative (IV) ); then hydrolyze it with 10% HCl acid (300ml) to obtain 20-hydroxy-2-bromoacetophenone (V). Finally, 5-chlorobenzofuran-3-one (A3) was obtained from the cyclization reaction of compound V and sodium acetate (0.6 mol) in ethanol under reflux conditions, filtered, and then crystallized: 87-89°C.
Apply[1]
5-Chloro-3-benzofuranone can be used to prepare 2-(3- or 4-hydroxybenzylidene) benzofuran-3-one derivatives:

(A3=5-chlorobenzofuran-3-one)
The specific steps are: reflux 5-chloro-3-benzofuranone (20mmol) in 70mL of n-butanol or isobutanol for 1 hour, add 3-(4-hydroxybenzaldehyde) derivative (22mmol) and use hydrochloric acid as catalyst (1 ml). The precipitate formed after cooling is filtered and crystallized. Obtain derivatives (C1-C10).
Main reference materials
[1]Synthesisandanti-canceractivityevaluationofnewauronederivatives

 微信扫一扫打赏
微信扫一扫打赏

