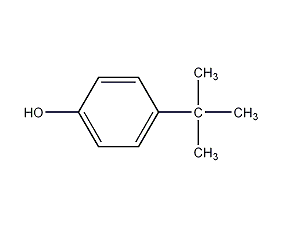
Structural formula
| Business number | 02E2 |
|---|---|
| Molecular formula | C10H14O |
| Molecular weight | 150 |
| label |
p-tert-butylphenol, 4-Hydroxy-1-tert-butylbenzene, 4-(1,1-dimethylethyl)phenol, Tert-butyl phenol, 4-Hydroxy-1-tert-butyl benzene, 4-(1,1-Dimethyl ethyl) phenol, Aromatic halogen derivatives |
Numbering system
CAS number:98-54-4
MDL number:MFCD00002367
EINECS number:202-679-0
RTECS number:SJ8925000
BRN number:1817334
PubChem ID:None
Physical property data
1. Characteristics: White needle-like crystals with a slight phenol odor. [1]
2. Melting point (℃): 98~99[2]
3. Boiling point (℃) :236~238[3]
4. Relative density (water=1): 0.91 (114℃)[4]
5. Relative vapor density (air=1): 5.1[5]
6. Saturated vapor pressure (kPa): 1.33 (114℃)[ 6]
7. Critical pressure (MPa): 3.34[7]
8. Octanol/water partition coefficient: 3.31[8]
9. Flash point (℃): 112.8[9]
10. Explosion limit (%): 5.8[10]
11. Lower explosion limit (%): 0.8[11]
12. Solubility: slightly Soluble in water, soluble in ethanol, ether, acetone, methanol and benzene. [12]
13. Refractive index (114ºC): :1.4787
Toxicological data
1. Skin/eye irritation:
Standard Dresser test: rabbit skin contact, 500mg/4HREACTION SEVERITY, slight reaction;
Standard Dresser test: rabbit Skin contact, 500mg/24HREACTION SEVERITY, mild reaction;
Standard Dresser test: rabbit eye contact, 10mgREACTION SEVERITY, strong reaction;
Standard Dresser test: rabbit eye contact , 50μg/24HREACTION SEVERITY, strong reaction;
2. Acute toxicity:
Rat oral LD50: 3250μL/kg;
Rat inhalation LCL0: 5600mg/m3/4H;
Mouse peritoneal cavity LD50: 78mg/kg;
Rabbit skin contact LD50: 2520μL/kg;
Mammalian oral LD50: 1500mg/kg;
Mammalian skin contact LD50: 1580mg/kg;
3. Chronic toxicity/carcinogenicity: hamster oral TDLo: 252mg/kg/20W-C
4. Acute toxicity [13]
LD50: 3250mg/kg (rat oral); 2520mg/kg (Rabbit transdermal)
5. Irritation [14]
Rabbit transdermal: 500mg (4h), Mild irritation.
Rabbit eye: 10mg, severe irritation.
Ecological data
1. Ecotoxicity[15]
LC50: 5.1mg/L (96h) (fathead minnow)
EC50: 4.2mg/L (24h) (Daphnia); 0.21mg/L (5min) (Photobacteria, Microtox toxicityTest)
2. Biodegradability No data available
3. Non-biodegradability No data available
4. Bioaccumulation [16] BCF: 34 (Chlorella)
Molecular structure data
1. Molar refractive index: 46.52
2. Molar volume (cm3/mol): 154.5
3. Isotonic specific volume (90.2K ): 370.3
4. Surface tension (dyne/cm): 32.9
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 18.44
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 115
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The phenolic woody and sweet aroma is a strong sensitizer and color remover. It can burn when exposed to open fire and decompose to release toxic gases.
2. Moderately irritating to eyes, skin and mucous membranes. Can burn skin when in hot melt state. At this time, you can wipe it with ethanol first, and then rinse it with plenty of warm water. Production equipment should be sealed and operators should wear protective equipment.
3. Stability[17] Stable
4. Incompatible substances[18] Bases, acid chlorides, acid anhydrides, oxidants, copper
5. Conditions to avoid contact [19] Heating
6. Hazards of aggregation[20] No aggregation
Storage method
1. Storage precautions[21] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. Packed in drums lined with plastic bags and fiberboard, each drum contains 25kg. Store and transport according to general flammable chemical regulations.
Synthesis method
1. Made from phenol and isobutylene as raw materials and cation exchange resin as catalyst.
2. Prepared by the reaction of phenol and diisobutylene. In addition to obtaining tert-butylphenol, the reaction process also co-produces p-octylphenol 4-(tert-Octyl)phenol, [140-66-9].
3. The finished product is obtained by reacting phenol with tert-butanol, washing with water, crystallizing, centrifuging and drying.
4. There are three process routes for tert-butylphenol.
(1) Made from phenol and isobutylene as raw materials.
Cationic ion exchange resin is used as a catalyst to perform catalytic alkylation in a fluidized bed at 80 to 140°C. The reaction content is 60% to 70%. The resulting mixed tert-butylphenol is catalytically translocated into pairs through a fixed bed. tert-butylphenol, and then distilled under reduced pressure to separate excess phenol for recycling, and obtain the product p-tert-butylphenol, with a yield of more than 90% based on isobutylene.
(2) It is obtained by the reaction of benzoic acid and 2,4,4-trimethyl-2-pentene (diisobutylene).
In addition to p-tert-butylphenol, p-octylphenol is also co-produced during the reaction.
(3) Obtained from the reaction of phenol and tert-butyl alcohol.
Mix anhydrous aluminum trichloride and petroleum ether, slowly add phenol while stirring, cool with ice water, then slowly add tert-butyl alcohol, stir for 15 minutes after addition, and then add anhydrous trichloride in batches Aluminum, stir for 4 to 5 hours, slowly add it to 1:1 hydrochloric acid, let it stand, separate the petroleum layer, extract the water layer with petroleum ether, combine the petroleum ether solution and wash it with water, separate the water and recover the petroleum ether, reduce Pressure distillation to obtain the finished product. When this process is used for industrial production, the synthesis reaction is carried out at 145 to 150°C.
![]()
Using heteropoly acid catalytic synthesis, add phenol and heteropoly acid catalyst H4SiW12O40·XH2O into the reactor together, heat to 110°C with constant stirring, and introduce a certain amount of isobutylene gas at a constant speed, reaction 4~ After 5 hours, pour out the reaction solution, wash with water, recrystallize, filter, and dry to obtain the finished product. The preferred reaction conditions are: reaction temperature 110°C, molar ratio of phenol to isobutylene 1:2, reaction time 4.5h, catalyst dosage accounting for 3% of the mass of phenol. Under these conditions, the product yield can reach 85%. %, compared with the sulfuric acid catalyzed synthesis of p-tert-butylphenol, the yield is increased by about 20%.
Purpose
1. Used in oil-soluble phenolic resin, which can be polycondensed with formaldehyde to obtain products with various uses. Mix 10-15% of this product into chloroprene adhesive to obtain soluble resin. This type of adhesive is mainly used in transportation, construction, civil engineering, shoemaking, etc. In terms of printing ink, it can be used for rosin modification, offset printing, advanced photogravure, etc. In terms of insulating varnishes, it can be used in coil impregnation varnishes and laminate varnishes, etc.
2. Used in polycarbonate production as a reaction terminator for phosgene polycarbonate. The added amount is 1-3% of the resin.
3. Used for modification of epoxy resin and xylene resin; used as polyvinyl chloride stabilizer, surfactant, and ultraviolet absorber.
4. It has antioxidant properties and can be used as a stabilizer for rubber, soap, chlorinated hydrocarbons and nitrocellulose. It is also a raw material for insect repellents (medicines), acaricides (pesticides), plant protection agents, spices, and synthetic resins. It can also be used as an additive for softeners, solvents, dyes, and paints. It can also be used as a component of demulsifiers for oil fields and as an additive for automobile oil.
5. It is an important raw material for the production of tert-butylphenol-formaldehyde resin. It is also used to synthesize oil-soluble phenolic resin, plasticizer for synthetic rubber, additive for paint, and used in medicine to produce insect repellent. wait. [22]
It is a reaction terminator for phosgene polycarbonate. The added amount is 1-3% of the resin.
3. Used for modification of epoxy resin and xylene resin; used as polyvinyl chloride stabilizer, surfactant, and ultraviolet absorber.
4. It has antioxidant properties and can be used as a stabilizer for rubber, soap, chlorinated hydrocarbons and nitrocellulose. It is also a raw material for insect repellents (medicines), acaricides (pesticides), plant protection agents, spices, and synthetic resins. It can also be used as an additive for softeners, solvents, dyes, and paints. It can also be used as a component of demulsifiers for oil fields and as an additive for automobile oil.
5. It is an important raw material for the production of tert-butylphenol-formaldehyde resin. It is also used to synthesize oil-soluble phenolic resin, plasticizer for synthetic rubber, additive for paint, and used in medicine to produce insect repellent. wait. [22]

 微信扫一扫打赏
微信扫一扫打赏

