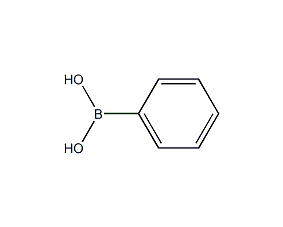
Structural formula
| Business number | 02EJ |
|---|---|
| Molecular formula | C6H7BO2 |
| Molecular weight | 121.93 |
| label |
Phenylboronic Acid, Phenyboric Acid, Benzeneboroic Acid, Pharmaceutical intermediates |
Numbering system
CAS number:98-80-6
MDL number:MFCD00002103
EINECS number:202-701-9
RTECS number:CY8575000
BRN number:970972
PubChem number:24887271
Physical property data
1. Character: white crystal
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1 ): Undetermined
4. Melting point (ºC): 217
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC) , 14mmHg): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation ( º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 40ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined Determined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17 . Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: in water and benzene It has little solubility in other solvents and is easily soluble in ether and methanol.
Toxicological data
1. Acute toxicity: rat oral LD50: 740mg/kg; mouse peritoneal cavity LD50: 500mg/kg; mouse intravenous injection LD50: 221mg/kg; dog intravenous LDLo: 450mg/kg; rabbit oral LDL0: 600mg /kg; rabbit skin contact LDL0: 4500mg/kg; guinea pig peritoneal cavity LDL0: 284mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 33.28
2. Molar volume (cm3/mol): 107.0
3. Isotonic specific volume (90.2K ): 271.7
4. Surface tension (dyne/cm): 41.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.19
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP):
2. Number of hydrogen bond donors: 2
3. Hydrogen bondingNumber of isomers: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polarity Surface area (TPSA): 40.5
7, Number of heavy atoms: 9
8, Surface charge: 0
9, Complexity: 79.1
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. The number of determined stereocenters of chemical bonds: 0
14. The number of uncertain stereocenters of chemical bonds: 0
15. The number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants.
2. The reagent is easily dehydrated when exposed to air or under heating conditions, forming a three-molecule polymer.
Storage method
1. Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and protected from moisture. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Within 1 hour, add 0.25 mol of phenylmagnesium bromide (approximately 1 mol of solution) to 58 g (0.25 mol) of n-butyl borateEster is dissolved in a solution of 100 ml of pure ether. During the reaction the solution was mechanically stirred and cooled in a solid carbon dioxide and acetone bath to maintain an internal temperature between -70°C and -75°C. When the Grignard reagent is dropped into the reaction solution, a white precipitate immediately forms, and the precipitate slowly dissolves. After the Grignard reagent is added, continue stirring the reaction mixture (-75°C) until the precipitate is completely dissolved. The resulting orange solution is slowly warmed to 0°C in a cold water bath (preferably overnight). Under vigorous stirring, the reaction mixture was slowly added to 150 ml of cold 10% sulfuric acid, and the ether layer was separated. The aqueous layer was extracted with 2 100 ml portions of diethyl ether. Combine the original ether layer and the ether extract, and evaporate the ether on a steam bath. The residual liquid contains phenylboric acid and n-butanol, which should be treated with potassium hydroxide solution until the solution becomes obviously alkaline. Add enough water to form about 150 ml of water layer below the alcohol layer. Heat gently under reduced pressure <not exceeding 45°C), steam out n-butanol by steam distillation, slowly add some warm water as needed, and continue distilling until there is no n-butanolUntil distillation. Finally, a small amount of biphenyl is evaporated, and a small amount of viscous solid separates from the water layer in the distillation flask.
Add sulfuric acid to the residual liquid to acidify it until the Congo red test paper changes color, and dilute (if necessary) to 150-200 ml. Without separating out the precipitate, heat the mixture to boiling while continuing to stir. After a short time, the crystals will completely melt and become a heavy, insoluble brown oil layer. Pour the aqueous layer through the pleated filter paper. Extract the oil layer with several portions of 20 ml boiling water, filter the extract while it is hot, and combine it with the original water layer. After cooling, white needle-shaped phenylboronic acid crystallizes. The product weighs 14-17 grams (50-60%) after drying and is no longer refined. The melting point is measured with a capillary tube and is 214-216°C (corrected). 1 to 2 grams of impure products can also be recovered from the mother liquor. When using more concentrated Grignard reagent solutions when preparing large quantities, the yield will be lower (42-47%).
Para-tolylboric acid, p-methoxyboric acid, etc. can be prepared using similar methods.
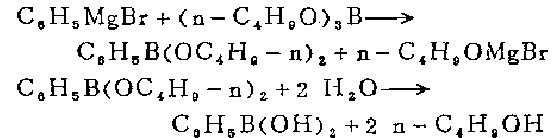
2. The laboratory can prepare it by reacting phenyl Grignard reagent and boric acid triester.
Purpose
Phenylboronic acid is a commonly used chemical reagent in chemistry laboratories. It can easily form cyclic borate esters (Formula 1) with compounds such as diols or diamines. When X = Y = O, the ability to form ester isn = 3 >2 > 4. The cis cyclic diol can form a 1:1 boronic acid ester with phenylboronic acid, while the trans cyclic diol can form a 1:2 polyester. This property can be used for the separation of cis- and trans- diols (Formula 2)[1].
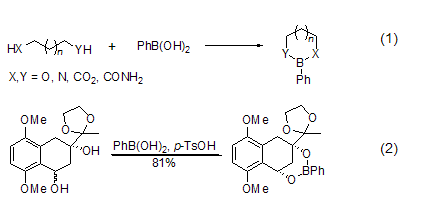
Phenylboronic acid easily mixes with Alcohols or diamines react and leave easily. Therefore, they are often used as protective groups for diols or diamine compounds[2]. This protective group is relatively stable in reactions such as esterification, methylation and silicon etherification of hydroxyl groups.
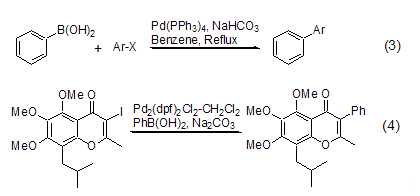
An important reaction in which phenylboronic acid participates is the Suzuki reaction. Under basic conditions and catalyzed by Pd complexes, phenylboronic acid can react with Vinyl-X (X = Br, OTf)[3], Ar-X (X = Cl[4 ], Br, I, OTf) coupling reaction occurs (Formula 3). As an important method for arylation, Suzuki reaction has been widely used in the total synthesis of some natural products (Formula 4)[5]. Phenylboronic acid is often used as a standard reactant to illustrate the advantages and disadvantages of different catalysts in the Suzuki reaction.
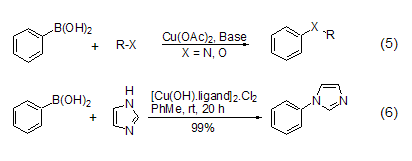
In Cu(OAc)2, phenylboronic acid can generate C-N bonds or C-O bonds (Formula 5) [6,7] to obtain aromatic amines, N em>-aryl substituted heterocyclic compound (formula 6)[8] or diaryl ether, etc. The reaction can proceed smoothly at room temperature using a weak base, which is a significant improvement over the classic Ulmmann arylation reaction and Goldberg reaction.
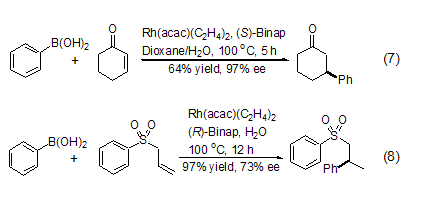
In metal Rh and chiral phosphorus Under the catalysis of ligands, organic boronic acids can undergo 1,4-asymmetric addition reactions with α,β-unsaturated carbonyl compounds. Compared with organometallic compounds, organoboronic acids not only have milder reaction conditions, but also have higher regioselectivity and enantioselectivity (Formula 7, Formula 8)[9,10].
em>-aryl substituted heterocyclic compound (formula 6)[8] or diaryl ether, etc. The reaction can proceed smoothly at room temperature using a weak base, which is a significant improvement over the classic Ulmmann arylation reaction and Goldberg reaction.

In metal Rh and chiral phosphorus Under the catalysis of ligands, organic boronic acids can undergo 1,4-asymmetric addition reactions with α,β-unsaturated carbonyl compounds. Compared with organometallic compounds, organoboronic acids not only have milder reaction conditions, but also have higher regioselectivity and enantioselectivity (Formula 7, Formula 8)[9,10].


 微信扫一扫打赏
微信扫一扫打赏

