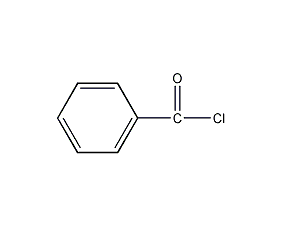
Structural formula
| Business number | 02ER |
|---|---|
| Molecular formula | C7H5ClO |
| Molecular weight | 140.57 |
| label |
benzoyl chloride, benzoyl chloride, Alpha-chlorobenzaldehyde, Benzenecarbonyl chloride;, Pesticide intermediates; aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:98-88-4
MDL number:MFCD00000653
EINECS number:202-710-8
RTECS number:DM6600000
BRN number:471389
PubChem number:24855444
Physical property data
1. Characteristics: colorless fuming liquid with pungent odor. [1]
2. Melting point (℃): -1[2]
3. Boiling point (℃): 197[3]
4. Relative density (water = 1): 1.22[4]
5. Relative vapor Density (air=1): 4.88[5]
6. Saturated vapor pressure (kPa): 0.13 (32.1℃)[6]
7. Heat of combustion (kJ/mol): -3272.1[7]
8. Critical pressure (MPa): 4.06[8]
9. Octanol/water partition coefficient: 1.440[9]
10. Flash point (℃): 72.2 [10]
11. Ignition temperature (℃): 185[11]
12. Explosion limit (%): 4.9 [12]
13. Lower explosion limit (%): 1.2[13]
14. Solubility: soluble in Ether, chloroform, benzene, carbon disulfide. [14]
15. Refractive index at room temperature (n20): 1.5537
16. Refractive index at room temperature (n25): 1.5515
17. Solubility parameter (J·cm-3)0.5: 20.387
18.van der Waals area (cm2·mol-1): 9.010×109
19. van der Waals volume (cm3·mol-1): 68.630
20. Gas phase standard claims heat (enthalpy) (kJ·mol -1): -10.32
21. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -158.0
22. Liquid phase standard hot melt (J·mol-1·K-1): 181.2
Toxicological data
1. Acute toxicity[15]
LD50: 1900mg/kg (rat oral); 790mg/kg (rabbit dermal )
LC50: 1870mg/m3 (rat inhalation, 2h)
2. Irritation No information available
3. Mutagenicity [16] Microbial mutagenicity: Salmonella typhimurium 1μmol/dish.
4. Carcinogenicity [17] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals.
Ecological data
1. Ecotoxicity[18]
LC50: 43mg/L (24h), 35mg/L (48h), 35mg/L (96h) (fathead minnow)
2. Biodegradability [19]
Aerobic biodegradation (h ): 168~672
Anaerobic biodegradation (h): 672~2688
3. Non-biodegradability[20]
Maximum light absorption of photolysis (nm): 293
Half-life of photooxidation in air (h): 102~1024
First-level hydrolysis half-life (h): 0.00472
Molecular structure data
1. Molar refractive index: 36.49
2. Molar volume (cm3/mol): 115.8
3. Isotonic specific volume (90.2K ): 291.1
4. Surface tension (dyne/cm): 39.8
5. Dielectric constant: 2.27
6. Dipole moment (10-24cm3):
7. Polarizability: 14.46
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 106
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. In case of open flame, high heat or contact with oxidants, there is a risk of combustion and explosion. It reacts with water to generate heat and releases toxic and corrosive gases. Corrosive.
2. Stability[21] Stable
3. Incompatible substances[22] Strong oxidants, strong alkalis, alcohols, water
4. Conditions to avoid contact [23] Humid air
5. Polymerization hazard[24] No polymerization
6. Decomposition products[25] Hydrogen chloride, phosgene
Storage method
1. Packed in glass bottles or porcelain jars. This product easily decomposes into benzoic acid and releases hydrogen chloride when exposed to water and air, so the packaging must be tight. Store in a dry and ventilated place. Heat and sun protection. Store and transport according to regulations on toxic substances.
2. Storage Precautions[26] Stored in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 25°C and the relative humidity does not exceed 75%. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, alcohols, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
The preparation methods are as follows.
(1) Phosgene method
Heat and melt benzoic acid, introduce phosgene at 140-150°C, pass in a certain amount to reach the end point, use nitrogen to drive out the phosgene, and the tail gas will be absorbed and destroyed, and the final product Obtained by distillation under reduced pressure.
(2) Phosphorus trichloride method
Dissolve benzoic acid in toluene and other solvents, add phosphorus trichloride dropwise, react for several hours after the drops are completed, distill off the toluene, and then evaporate. Deliver finished product.
(3) Trichloromethylbenzene method
Carry out side chain chlorination of toluene and then hydrolyze it to obtain the product.
(4)Toluene and chlorine undergo side chain chlorination reaction under light conditions to obtain trichlorotoluene, which is then hydrolyzed in acidic medium. Or derived from the reaction of benzoic acid and light. It can also be obtained by reacting benzoic acid with silicon tetrachloride.
(5)Use silicon tetrachloride and p-benzoic acid to acid chloride, and then distill and refine.
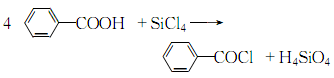
Purpose
1. It can be used in jelly fracturing fluid cross-linked with non-ionic vegetable gum and amphoteric metal oxygen acid salts. Generally not used alone, but often used in combination with persulfate. It is also used in the production of dye intermediates, benzophenone UV absorbers, rubber additives, medicines, etc.
2. Used as analytical reagent. Used in organic synthesis, spice preparation, dye intermediates, etc. It is used to produce ice dye blue base BB, blue base RR, purple base B and direct lightfast red GB. It can also produce reduced 5GK, reduced olive R, reduced orange 3G, reduced gray BGG, red 4B, and dispersed yellow SE. -2GL, yellow RFL, dispersed fluorescent yellow FFL, etc.
3. Used in medicine and organic synthesis. [27]

 微信扫一扫打赏
微信扫一扫打赏

