Background and overview[1]
2,5-Dichlorophenylethylamine can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
2,5-Dichlorophenylethylamine was prepared as follows: To a solution of 2-(2,5-dichlorophenyl)acetonitrile (34.5g, 185mmol) in MeOH (1700mL) at 0°C, NiCl2 was added ·6H2O (4.45g, 18.7mmol). After 10 minutes, the solids from the initial green suspension went into solution. Methyl chloroformate (38.4 g, 406 mmol) was added via syringe over 10 min, followed by NaBH4 (41.4 g, 1.09 mol) in 8 small portions over 30 min at 0 °C. The evolution of gas was observed and the reaction mixture turned black. After the addition was complete, the resulting mixture was stirred at 20°C for 1 hour. LCMS analysis detected the desired product in 48% yield and 2,5-dichlorophenylethylamine in 32% yield.
Apply[1]
2,5-Dichlorophenylethylamine can prepare the following compounds:
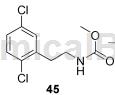
To 2,5-dichlorophenylethylamine was added methyl chloroformate (7.7g, 81mmol) via syringe at 20°C. The resulting mixture was stirred at 20°C for 1 hour. The mixture was quenched with H2O (100 mL) and concentrated to give an off-white residue. Partition the solid between EtOAc (1500 mL) and aqueous NaOH (1 N, 400 mL). The organic layer was separated and the resulting aqueous suspension layer was filtered. The filtrate was extracted with EtOAc (2×300 mL). The organic extracts were combined, washed with brine (150 mL) and dried over Na2SO4. Concentration gave crude product (40g), which was purified by column chromatography (petroleum ether/EtOAc=10:1 to 4:1, Rf=0.3 in petroleum ether/EtOAc=10:1) to give 45 (25g, 54% ) is a yellow solid. LCMSm/z248[M+H]+; 1HNMR (400MHz, DMSO-d6) δppm7.45 (d, J=8.6Hz, 1H), 7.39 (d, J=2.3Hz, 1H), 7.32 (dd, J= 2.6, 8.6Hz, 1H), 7.21 (t, J=4.3Hz, 1H), 3.50 (s, 3H), 3.23 (q, J=6.6Hz, 2H), 2.83 (t, J=7.0Hz, 2H)
Main reference materials
[1] Design and Synthesis of Pyridone-Containing 3,4- Dihydroisoquinoline-1(2H)‑ones as a Novel Class of Enhancer of Zeste Homolog 2 (EZH2) Inhibitors

 微信扫一扫打赏
微信扫一扫打赏

