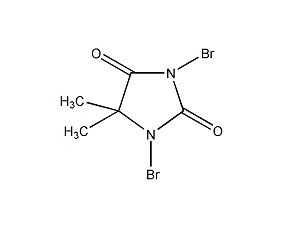
Structural formula
| Business number | 01LT |
|---|---|
| Molecular formula | C5H6Br2N2O2 |
| Molecular weight | 285.92 |
| label |
1,3-dibromo-5,5-dimethyl-2,4-imidazolidinedione, Dibromodimethylhydantoin, 1,3-dibromo-5,5-dimethylhydantoin, dibromohydantoin, 1,3-Dibromo-5,5-dimethylcaprolhydantoin, 1,3-Dibromo-5,5-dimethylureidoacetolactam, 5,5-Dimethyl-1,3-dibromohydantoin, Hydantoin, 1,3-dibromo-5,5-dimethyl-, Dibromantine, 1,3-Dibromo-5,5-dimethyl-2,4-imidazolidinedione, Halogenating reagent |
Numbering system
CAS number:77-48-5
MDL number:MFCD00003189
EINECS number:201-030-9
RTECS number:MU0686000
BRN number:146024
PubChem number:24849707
Physical property data
1. Characteristics: light yellow crystal.
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):197 -199℃
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index: Uncertain
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
(10-24cm3):18.28
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 40.6
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 229
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
2 Bromohydantoin removal has the high efficiency, broad spectrum, wide range of application, small residue, and is not affected by ammonia nitrogen/PHIn addition to its advantages such as value influence, dibromohydantoin also has advantages and characteristics that other halogenated hydantoins do not have: dibromohydantoin is mainly formed when hydrolyzed in water Hypobromous acid, which releases bromine in the form of hypobromous acid. The reaction of dibromohydantoin to release bromine occurs quickly, and a large amount of bactericidal hypobromous acid is quickly formed in the water. However, other halogenated hydantoins, such as dichlorohydantoin or bromochlorohydantoin, react slowly to release halogen ions in the water. The peak time for disinfectant sterilization is relatively delayed, so dibromohydantoin is more efficient than other halogenated hydantoins under conditions that require relatively rapid sterilization.
Storage method
Storage In a cool, dry environment, it is strictly prohibited to mix it with toxic and harmful substances to avoid contamination.
Synthesis method
By 5,5-Dimethylpyridine Urea is obtained by bromination. Will5,5- Dimethyl B Mix hydantoin, sodium carbonate, sodium hydroxide and water, slowly add bromine while cooling, pour into water after addition, and filter out the crystals after standing, which is the crude product. Then dissolve the crude product in acetone, decolorize and filter, and pour the filtrate into water to obtain white crystals, which is the finished product.
Purpose
This It is a new type of sterilizing and disinfecting water treatment agent. Widely used in industrial water treatment systems, indoor and outdoor swimming(Bath) Pool, health system and disinfection of breeding water environment. Can kill a variety of germs and viruses. It can prevent and treat aquaculture animal diseases caused by bacteria and viruses.
�Disinfection of the environment. Can kill a variety of germs and viruses. It can prevent and treat aquaculture animal diseases caused by bacteria and viruses.

 微信扫一扫打赏
微信扫一扫打赏

