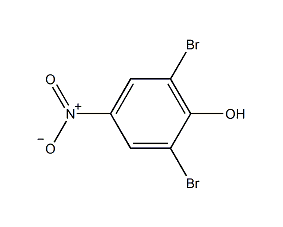
Structural formula
| Business number | 02FH |
|---|---|
| Molecular formula | C6H3Br2NO3 |
| Molecular weight | 296.9 |
| label |
2,6-dibromo-4-nitrophenol, 4-nitro-2,6-dibromophenol, 2,6-Dibromo-4-nitro-pheno, 4-Nitro-2,6-dibromophenol, Phenol, 2,6-dibromo-4-nitro-, 2,6-Dibromo-4-nitrophenol, 2,6-Dibromo-4-mitrophenol, 2,6-Dibromo-4-nitrophenol, 99+%, Dibromo-4-nitrophenol, 2,6- |
Numbering system
CAS number:99-28-5
MDL number:MFCD00007334
EINECS number:202-744-3
RTECS number:SK8025000
BRN number:1245050
PubChem number:24893846
Physical property data
1. Properties: yellow columnar crystals.
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 145
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 98.5KPa): Undetermined
p>
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 119ºC): Not determined
12. Saturated vapor pressure ( kPa, 119.3ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient:
17. Explosion upper limit (%, V/V ): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ether, carbon disulfide, ethyl acetate, chloroform and heat Alcohol, insoluble in benzene, acetic acid and petroleum ether, almost insoluble in water.
Toxicological data
Acute toxicity: intravenous injection in mice LD50: 56 mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 50.05
2. Molar volume (cm3/mol): 132.0
3. Isotonic specific volume (90.2K ): 378.7
4. Surface tension (dyne/cm): 67.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 19.84
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 66
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 172
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Obtained from bromination of p-nitrophenol. Mix p-nitrophenol and glacial acetic acid and stir to dissolve them all. Slowly add the mixed solution of bromine and glacial acetic acid at 20-25°C. Complete the addition in about 3 hours. After the addition is completed, stir for 1 hour and keep at 85°C for 1 hour. Combine under pressure to drive off the bromine, add water and stir to precipitate, cool for 6 hours, filter, wash the crystals with 50% acetic acid, then wash with water, and dry at 40-60°C to obtain a crude product. Then recrystallize with acetic acid to obtain the finished product.
Purpose
As an intermediate in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

