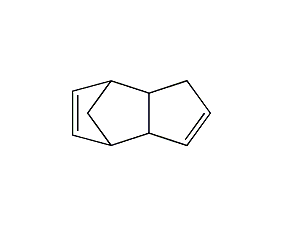
Structural formula
| Business number | 01M1 |
|---|---|
| Molecular formula | C10H12 |
| Molecular weight | 132.20 |
| label |
dicyclopentadiene, dicyclopentadiene, 4,7-methylene-4,7,8,9-tetrahydroindene, cyclopentadiene, cyclopentadiene dimer, diphenene, Dicyclopentadiene, dicyclopentadiene, 4,7-methylene-4,7,8,9-tetrahydro-indene, cyclopentadiene, cyclopentadiene dimer, two RB-ene, paint; adhesive, Ester cyclic compounds and their derivatives |
Numbering system
CAS number:77-73-6
MDL number:MFCD00078246
EINECS number:201-052-9
RTECS number:PC1050000
BRN number:1904092
PubChem number:24868570
Physical property data
1. Properties: Colorless crystals, with an odor similar to camphor
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1)::0.979
4. Melting point (ºC): 175℃
5. Boiling point (ºC, normal pressure): 170℃
6. Boiling point (ºC, 5.2kPa): 88℃ (4.67kPa)
7. Refractive index: 1.5061
8. Flash point (ºC): 26.7~37.7
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition Temperature (ºC): 680℃
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: soluble in alcohol, ether and carbon tetrachloride, insoluble in water
Toxicological data
Toxicity classification Highly toxicAcute toxicity Oral – Rat LD50: 353 mg/kg; Oral – Mouse LD50: 190 mg/kg. Irritation data Skin – Rabbit 10 mg/24 hours Severe; Eyes – Rabbit 500 mg/24 hours Mild.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 41.42
2. Molar volume (cm3/mol): 126.0
3. Isotonic specific volume (90.2K ): 320.6
4. Surface tension (dyne/cm): 41.8
5. Dielectric constant (F/m): 2.29
6. Extreme Chemical rate (10-24cm3): 16.42
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 212
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 4
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Colorless crystals with an odor similar to camphor. Soluble in organic solvents such as ethanol and ether, insoluble in water, and easy to undergo addition reactions and self-polymerization reactions.
2.This product is toxic, has paralyzing and local irritation effects, and acute poisoning can cause coma. The oral LD50 of rats is 820mg/kg body weight, and the skin absorption LD50 of rabbits is 6.72mL/kg body weight. The main pathological changes of oral poisoning in rats are extensive congestion and bleeding in the lungs, stomach, intestines, kidneys and bladder. Devices and equipment containers should be sealed and ventilation should be enhanced. The maximum allowable concentration in the air is
5 mg/m3. Operators should wear protective equipment. Those who experience acute poisoning should be moved to fresh air and treated according to general narcotic drug poisoning.
Storage method
Stored in a sealed and cool, ventilated warehouse at room temperature. Keep away from fire and heat sources.
This product is packed in small iron drums and stored in a cool and ventilated place. Store and transport according to regulations on toxic, flammable and dangerous goods.
Synthesis method
The light benzene fraction of coal tar is distilled, and the benzene head fraction of <70°C is cut, which contains about 30% cyclopentadiene. It is then heated, polymerized, and distilled to obtain the finished product dicyclopentadiene. It is obtained by using the C5 fraction in the hydrocarbon cracking industry as raw material, dimerizing it by heating, and then performing vacuum distillation and separation. Secondly, it can also be obtained by dimerization using cyclopentadiene as raw material. Process flow: (1) Using C5 fraction as raw material, first heat the C5 fraction to 80-120°C to dimerize cyclopentadiene. Then, rectification is performed, and the dicyclopentadiene fraction is separated from the bottom of the tower. Because in this fraction, in addition to cyclopentadiene, isoprene, etc., dienes are also mixed. When it is heated to above 170°C, only dicyclopentadiene will be decomposed and distilled first. The obtained cyclopentadiene is subjected to thermal polymerization and distillation to obtain dicyclopentadiene. (2) Thermal polymerization can be used to convert cyclopentadiene into a dimer with a boiling point of 170°C, and then purified by distillation. The dimerization reaction proceeds most completely at 70°C but the reaction process is very slow. Use heating and total reflux for 16-18 hours, then raise the temperature to take the front fraction (below 82°C) and benzene fraction (above 82°C) until the kettle temperature reaches 110°C. The residue at the bottom of the still is dicyclopentadiene, with a content of about 60%. After further distillation under reduced pressure, a dimer with a content of ≥99% can be obtained. ![]()
Purpose
The main uses of cyclopentadiene dimer (or cyclopentadiene) are: (1) used as the third component of ethylene-propylene binary copolymer (EPDM); (2) used to synthesize ethylene Raw material of norbornene dianhydride (ENB); (3) React with maleic anhydride to obtain norbornene dianhydride, which is used as raw material for epoxy resin curing agent, polyester resin, alkyd resin, and pesticide; ( 4) The hexachloride of cyclopentadiene – hexachlorocyclopentadiene, the hydrogenated phthalic anhydride of hexachlorinated endomethylene obtained by reacting with maleic anhydride is used as a flame retardant for synthetic resins; ( 5) Dipolycyclopentadiene is used to modify tung oil, linseed oil, soybean oil, fish oil, etc., which can speed up drying and improve water resistance and alkali resistance; (6) Dipolycyclopentadiene resin can be used as Rubber adhesives, pressure-sensitive adhesives, hot melt adhesives, inks, coatings, etc.; (7) Dipolycyclopentadiene oxide can be used as injection molding and lamination molding resin; (8) Cyclopentadiene Cyclopentene obtained by partial hydrogenation of olefin is then polymerized to obtain 1,5-trans polycyclopentene; (9) Cyclopentadiene is a raw material for the production of aldrin and dildrin pesticides; (10) Cyclopentadiene is also used in drug synthesis. For example, Weichangle’s intermediate α-cyclopentadienylmandelate methyl ester is obtained by the addition of methyl acetophenonate and cyclopentadiene; (11) it is used as a high-energy fuel after hydrogenation.
Monomer for polymerization reaction. Used in the manufacture of synthetic rubber adhesives, medicines, pesticides, synthetic spices and other products. The copolymer with maleic acid can be used as coatings and adhesives; the copolymer with maleic anhydride can be used as paper sizing agent; the copolymer with terpene oil can be made into offset printing ink; with piperylene Copolymers can be used as rubber reinforcing agents. The tetrahydride of this product is a high-energy fuel for rockets and jet engines.
, obtained by the addition of methyl acetophenonate and cyclopentadiene; (11) used as high-energy fuel after hydrogenation.
Monomer for polymerization reaction. Used in the manufacture of synthetic rubber adhesives, medicines, pesticides, synthetic spices and other products. The copolymer with maleic acid can be used as coatings and adhesives; the copolymer with maleic anhydride can be used as paper sizing agent; the copolymer with terpene oil can be made into offset printing ink; with piperylene Copolymers can be used as rubber reinforcing agents. The tetrahydride of this product is a high-energy fuel for rockets and jet engines.

 微信扫一扫打赏
微信扫一扫打赏

