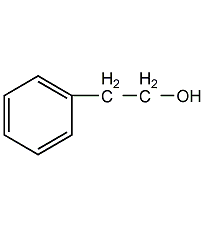
Structural formula
| Business number | 01B1 |
|---|---|
| Molecular formula | C8H10O |
| Molecular weight | 122.17 |
| label |
phenethyl alcohol, β-phenylethyl alcohol, α-Phenylethanol, benzylmethanol, Phenethyl alcohol, β-Phenylethyl alcohol, α-Phenyl ethanol, benzylcarbinol, For preparing soap, cosmetic fragrance, food flavors, alcohol solvent |
Numbering system
CAS number:60-12-8
MDL number:MFCD00002886
EINECS number:200-456-2
RTECS number:SG7175000
BRN number:1905732
PubChem number:24887136
Physical property data
1. Properties: Colorless liquid with a faint rose scent.
2. Boiling point (ºC, 101.3kPa): 218.2
3. Boiling point (ºC, 1.33kPa): 219~221 (100kpa)
4. Melting point (ºC): -27
5. Relative density (d254) : 1.023
6. Refractive index (n20ºC): 1.5325
7. Viscosity (mPa·s, 25ºC): 7.58
8. Viscosity (mPa·s, 50ºC): 3.19
9. Flash point (ºC): 102.2
10. Vapor pressure (kPa, 58ºC): 0.1333
11. Relative vapor density (g/mL, air=1): 4.22
12. Solubility: Soluble in water, freely miscible with ethanol, ether and glycerin, solubility in water is 1:50.
13. Relative density (20℃, 4℃): 1.0202
14. Relative density (25℃, 4℃): 1.0162
15. Normal temperature Refractive index (n25): 1.5305
16. Liquid phase standard hot melt (J·mol-1·K-1):232.1
Toxicological data
1. Acute toxicity: Oral – Rat LD50: 1790 mg/kg.
2. Irritation data: skin – rabbit 100 mg/24 hours moderate; eyes – rabbit 0.75 mg/24 hours severe.
3. It is of low toxicity. It has anesthetic effect and is mildly irritating to the skin.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 37.33
2. Molar volume (cm3/mol): 119.7
3. Isotonic specific volume (90.2K): 300.6
4. Surface tension (dyne/ cm): 39.6
5. Polarizability (10-24cm3): 14.80
Compute chemical data
1. Reference value for calculation of hydrophobic parameters (XlogP): 1.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
p>
4. Number of rotatable chemical bonds: 2
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 20.2
p>
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 65
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Reaction with aromatic alcohols. The hydroxyl group can be replaced by halogen and esterified by all organic acids in the presence of sulfuric acid. Aromatic rings can be nitrated, sulfonated and halogenated. Hydrogenation produces cyclohexylethanol.
2.This product has stimulating and anesthetic effects on rats, with an acute oral LD50 of rats, mice and mice. They are 1700mg/kg, 800mg/kg and 400mg/kg respectively. However, it is allowed to be used in food in the United States, and is used in artificial flavors in Europe at a concentration of 2 mg/kg. The irritation and allergy tests for humans are negative.
3. Exists in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
4. Naturally found in rose, geranium, ylang ylang, orange blossom and other plant essential oils.
5. Recognized as GRAS by FEMA and approved for use by FDA.
Storage method
1. This product should be sealed and stored away from light.
2. Packed in glass bottles, wrapped in wooden barrels or plastic barrels, and stored in a cool, dry and ventilated place. Protect from sun and moisture, and keep away from fire and heat sources. Store and transport according to general chemical regulations. When transporting, pack and unload with care to avoid damage to the packaging
Synthesis method
Prepared from styrene monomer by oxidizing styrene. Or it can be obtained by reacting benzene with ethylene oxide.
1. Styrene oxide method is obtained by hydrogenating styrene oxide at low temperature and pressure in the presence of a small amount of sodium hydroxide and a skeleton nickel catalyst.
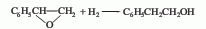
2. Ring Oxyethane method is produced by the Friedel-Crafts reaction of benzene and ethylene oxide in the presence of anhydrous aluminum trichloride.
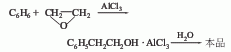
3. Phenylethyl alcohol exists naturally in rose oil, ylang-ylang oil, neroli oil, hyacinth oil, etc. Most commercially available products are prepared by synthetic methods. Industrially, styrene is reacted with NaBrO, then HBr is removed and cyclized, and finally phenylethanol is obtained by catalytic hydrogenation; or phenylethanol is obtained by reacting benzene and ethylene oxide in anhydrous aluminum chloride as a catalytic reaction.
4. Tobacco: BU, 56; OR, 57; BU, 14; FC, BU, OR, 18; OR, 41; FC, BU, OR, 40.
5. Preparation method:
![]()
In a reaction bottle equipped with a stirrer and a reflux condenser, add 0.6g (5mmol) of styrene epoxide (2), 0.63g (10mmol) of ammonium formate, and 0.05% Pd-C catalyst g, 15 mL of methanol, stir and reflux for 2 hours. Check with TLC during this period. After the reaction is completed, cool and filter, and the filtrate is concentrated under reduced pressure to obtain 0.61 g of compound (1)①, with a yield of about 100%. Note: ① The following various alcohol compounds (Table I-5-2) can be synthesized using similar methods. Reference book page 173. [1]
6. Preparation method:
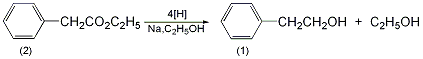
In a 3L reaction bottle, add 120mL of metal-dried toluene and 42g of metal sodium, stir vigorously Make sodium sand and cool it to 60℃ without pouring out toluene. Quickly add a solution composed of 50g (0.3mol) of ethyl phenylacetate (2) and 190mL of absolute ethanol at a speed that does not make the reaction too violent and uncontrollable. Then add 250 mL of absolute ethanol. After the reaction is stable, the water bath is heated until all sodium metal has reacted. The ethanol and toluene were distilled off under reduced pressure. Water was added to the residue, and after cooling, the mixture was extracted with diethyl ether. The extract was dried over anhydrous magnesium sulfate. First, evaporate the diethyl ether, and then distill under reduced pressure. Collect the fraction at 116~118℃/3.3kPa to obtain 25g of 2-phenylethanol (1), with a yield of 67%. [2]
Purpose
1. Blending rose-scented flower essential oil and various floral flavors, such as jasmine, lilac, orange blossom, etc. It can blend almost all flower essential oils and is widely used in blending soap and cosmetic flavors. In addition, various food flavors can also be formulated, such as strawberry, peach, plum, melon, caramel, honey, cream and other food flavors. Used in the manufacture of spices, cosmetics, preservatives and other raw materials.
2.Phenylethyl alcohol is an edible spice allowed to be used according to my country’s hygienic standards for food additives. The dosage is based on normal production needs, generally 21 to 80 mg/kg in chewing gum; 12 mg/kg in candies. mg/kg; in baked goods 16mg/kg; in cold drinks 8.3 mg/kg.
A variety of food flavors, such as strawberry, peach, plum, melon, caramel, honey, cream and other food flavors. Used in the manufacture of spices, cosmetics, preservatives and other raw materials.
2.Phenylethyl alcohol is an edible spice allowed to be used according to my country’s hygienic standards for food additives. The dosage is based on normal production needs, generally 21 to 80 mg/kg in chewing gum; 12 mg/kg in candies. mg/kg; in baked goods 16mg/kg; in cold drinks 8.3 mg/kg.

 微信扫一扫打赏
微信扫一扫打赏

