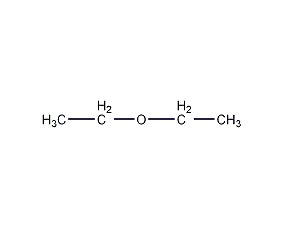
Structural formula
| Business number | 01B8 |
|---|---|
| Molecular formula | C4H10O |
| Molecular weight | 74.12 |
| label |
ethoxyethane, diethyl ether, Diethyl ether, Ether, Ethoxyethane, Ethyl oxide, Anesthetic ether, Dehydrating solvents for organic synthesis, organic acid extractant, Anesthetic, Ether solvent |
Numbering system
CAS number:60-29-7
MDL number:MFCD00011646
EINECS number:200-467-2
RTECS number:KI6775000
BRN number:1696894
PubChem number:24857834
Physical property data
1. Properties: colorless and transparent liquid with aromatic odor and highly volatile. [1]
2. Melting point (℃): -116.2[2]
3. Boiling point (℃): 34.6[3]
4. Relative density (water=1): 0.71 (20℃)[4]
5. Relative vapor density (air = 1): 2.56[5]
6. Saturated vapor pressure (kPa): 58.92 (20℃)[6]
7. Heat of combustion (kJ/mol): -2748.4[7]
8. Critical temperature (℃): 192.7[8]
9. Critical pressure (MPa): 3.61[9]
10. Octanol/water partition coefficient: 0.89[10]
11. Flash point (℃): -45 (CC) [11]
12. Ignition temperature (℃): 160~180[12]
13. Explosion upper limit (%): 49.0[13]
14. Lower explosion limit (%): 1.7[14]
15. Solubility: slightly soluble in water, soluble in ethanol, benzene, chloroform, solvent naphtha Most organic solvents such as oil. [15]
16. Melting point (ºC, stable type): -116
17. Melting point (ºC, unstable type): -116.3
18. Relative density (g/mL, 0/4ºC): 0.7364
19. Relative density (g/mL, 10/4ºC): 0.7249
20. Relative density (g/mL, 25/4ºC): 0.706
21. Relative density (g/mL, 30/4ºC): 0.7019
22. Refractive index (n20ºC ): 1.3524
23. Refractive index (n25ºC): 1.3495
24. Viscosity (mPa·s, 20ºC): 0.2448
25. Viscosity (mPa ·s, 25ºC): 0.2230
26. Heat of evaporation (KJ/mol, 30ºC): 26.02
27. Heat of fusion (KJ/kg): 98.53
28. Heat of formation (KJ/kg, 25ºC): -272.98
29. Heat of combustion (KJ/kg, 20ºC): 2728.53
30. Specific heat capacity (KJ/( kg·K), 0ºC, constant pressure): 2.25
31. Specific heat capacity (KJ/(kg·K), 30ºC, constant pressure): 2.30
32. Specific heat capacity (KJ /(kg·K), 120ºC, constant pressure): 3.36
33. Specific heat capacity (KJ/(kg·K), 180ºC, constant pressure): 4.36
34. Boiling point Rise constant: 21.6
35. Conductivity (S/m, 25ºC): 3.7×10-13
36. Volume expansion coefficient (K-1): 0.00164
37. Body expansion coefficient (K-1, 0~1L concentrated sulfuric acid, mix evenly, and then add a few grains of zeolite. Heat to 140°C with an electric heating mantle. Add 100mL of 95% ethanol (2) dropwise from the dropping funnel, control the dropping speed to be basically the same as the elution speed of ether (1-2 drops/second), and keep the liquid temperature between 135-140°C, add for about 3 hours over. After the addition is completed, the heating reaction is continued for 10 minutes. When the temperature rises to 160°C, the reaction is stopped. Wash the distilled solution with 5% sodium hydroxide aqueous solution, saturated sodium chloride solution, saturated calcium chloride solution ① in sequence, and dry over anhydrous calcium chloride. Heating and distilling in a water bath, collecting the fractions at 33-38°C to obtain 40g of diethyl ether②(1), with a yield of 48%. Note: ① When washing with saturated calcium chloride solution and drying with calcium chloride, in addition to removing water, a small amount of ethanol can also be removed, because ethanol can form a complex with calcium chloride.
② Ether (including other ethers Compounds) are easily oxidized to form peroxides. Peroxides are prone to danger during distillation. Therefore, in some reactions or before distillation, the presence of peroxide should first be checked and removed. For specific methods, see the appendix Preparation and Purification Methods of Commonly Used Organic Solvents and Reagents. [27]
Purpose
1. Used as a solvent and an important anesthetic in medicine. Mixed with ethanol to dissolve nitrocellulose in the production of smokeless gunpowder, cotton glue and photographic films. It can be used as a solvent for grease, resin, wax, rubber, alkaloids, organic metal compounds, etc. Mixtures with ethanol are excellent solvents for nitrocellulose. In addition, it is also used as organic synthesis solvent, organic acid extractant and anesthetic.
2. Analytical reagents, used for the analysis of nickel, potassium, magnesium, etc. Used as solvents, cleaning agents, pharmaceuticals, electronics and other industries.
3. Used as analytical reagents, such as solvents, extractants, liquid chromatography eluents and solvents. Also used as an anesthetic.
4. Used as a solvent and an anesthetic in medicine. [26]

 微信扫一扫打赏
微信扫一扫打赏

