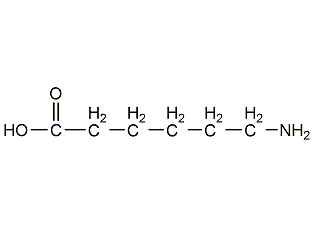
Structural formula
| Business number | 01BA |
|---|---|
| Molecular formula | C6H13NO2 |
| Molecular weight | 131.17 |
| label |
Aminocaproic acid, ε-Aminocaproic acid, 6-Aminohexanoic acid, EACA, Afibrin, Amicar, Epsilcapramin, Hemocaprol, Ipsilon |
Numbering system
CAS number:60-32-2
MDL number:MFCD00008238
EINECS number:200-469-3
RTECS number:MO6300000
BRN number:906872
PubChem number:24890662
Physical property data
1. Properties: White leaf-like crystals. Odorless. Bitter taste.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 203-204
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, slightly soluble in Methanol, insoluble in ethanol and ether.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 34.94
2. Molar volume (cm3/mol): 125.8
3. Isotonic specific volume (90.2K): 321.6
4. Surface tension (dyne/cm): 42.6
5. Polarizability (10-24cm3): 13.85
Compute chemical data
1. Hydrophobic parameter calculation parameters�� value (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Yes Number of rotating chemical bonds: 5
5. Number of tautomers: None
6. Topological molecule polar surface area 63.3
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 83.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Irritating.
Storage method
Store sealed and protected from light.
Synthesis method
Derived from the hydrolysis of caprolactam. Add caprolactam, concentrated hydrochloric acid and 3 times the amount of distilled water into the reaction tank, stir, raise the temperature and reflux for 1.5 hours, and control the temperature to 103-106°C. After the reaction is completed, the measured conversion rate should be above 95%. Add distilled water to dilute to 10% concentration to obtain 10% 6-aminocaproic acid hydrolyzate. Flow the hydrolyzate evenly into the ion exchange column [packed with 001×7 (732#) strongly acidic styrene-based cation exchange resin] at a flow rate of 5-10L per hour for adsorption. After the flow is finished, close the outlet and soak overnight. Wash the ion exchange column with distilled water and remove the hydrochloric acid (check the effluent to ensure there is no chloride ions). Then, flow 3.5% ammonium hydroxide evenly at a flow rate of 5-10L per hour. After the flow is finished, close the outlet and soak overnight. After continuing to finish ammonium hydroxide the next day, flow into distilled water for elution, and collect the eluate containing 6-aminocaproic acid. Add activated carbon to decolorize, filter, and concentrate the filtrate under reduced pressure at 47-70°C to nearly dryness. Add ethanol while hot and stir to precipitate crystals. After cooling, shake off and filter to obtain the crude product. Dissolve the crude product in distilled water, add activated carbon and decolorize at 60°C for 1 hour. Strain while hot. The filtrate was concentrated under reduced pressure to nearly dryness. Add ethanol to precipitate crystals, cool and filter, wash the crystals with ethanol and dry to obtain 6-aminocaproic acid. The overall yield of caprolactam is 75-80%.
Purpose
1. Biochemical research. Hemostatic agent. Organic Synthesis. Synthetic nylon.

 微信扫一扫打赏
微信扫一扫打赏

