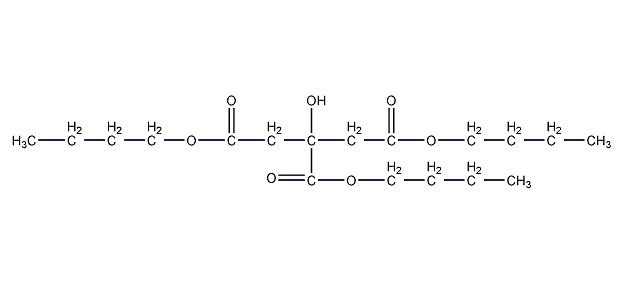
Structural formula
| Business number | 01MH |
|---|---|
| Molecular formula | C18H32O7 |
| Molecular weight | 360.44 |
| label |
tributyl 2-hydroxy-1,2,3-propanetricarboxylate, Tri-n-butyl citrate, Tributyl 2-Hydroxypropyltrihydroxyacid, Tributyl acid ester, Tributyl 2-hydroxypropionic acid carboxylate, butyl citrate, Tri-n-butyl citrate, Citric acid tri-n-butyl ester, 2-Hydroxy-1,2,3-propanetricarboxylic acid tributyl ester, Tributyl 2-hydroxy-1,2,3-propanetricarboxylate, plasticizer, defoamer, Rust inhibitor |
Numbering system
CAS number:77-94-1
MDL number:MFCD00027217
EINECS number:201-071-2
RTECS number:TZ8608000
BRN number:1806072
PubChem number:24856617
Physical property data
1. Appearance: Colorless transparent oily liquid
2. Density (g/mL, 25/25℃): 1.042
3. Relative vapor density (g/mL , air=1): Uncertain
4. Melting point (ºC): -20
5. Boiling point (ºC, normal pressure): 225
6 . Boiling point (ºC, 0.13kPa): 169~170
7. Refractive index (25ºC): 1.4431
8. Flash point (ºC, open): 182
9. Viscosity (mPa·s, 25ºC): 31.9
10. Flash point (ºC): 368
11. Solubility (%, 25ºC, water): <0.002
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Insoluble in water, soluble in Methanol, acetone, carbon tetrachloride, glacial acetic acid, castor oil, mineral oil and other organic solvents.
Toxicological data
1. Acute toxicity: mouse abdominal LD50: 2900 mg/kg;
2. Other multiple dose toxicity data: mouse abdominal LD50: 8120mg/kg/14D-I
3. Low toxicity, easy to useRats fed a diet of 5% tributyl citrate for 6 weeks had no effect on growth. Rat oral LD50: >30mL/kg, cat oral LD50: >50mL/kg.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 92.26
2. Molar volume (cm3/mol): 333.6
3. Isotonic specific volume (90.2K ): 834.2
4. Surface tension (dyne/cm): 39.0
5. Polarizability (10-24cm3): 36.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.7
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 7
4. Number of rotatable chemical bonds: 17
5. Number of tautomers: none
6. Topological molecule polar surface area 99.1
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 382
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Soluble in most organic solvents. This product has low volatility, good compatibility with resin, and high plasticizing effect. It can give products good cold resistance, water resistance and mildew resistance.
Chemical properties: It has the chemical properties of general esters, but is more stable. Only 0.1% of citric acid is freed after heating at 150°C for 1 hour. Hydrolysis can occur in the presence of caustic alkali.
Storage method
Should be stored in a ventilated and dry place indoors, away from sources of fire. Store and transport according to regulations on flammable chemicals.
Synthesis method
1. Citric acid and n-butanol (feeding ratio 1:1) are refluxed at 150°C for 4 to 5 hours under the action of sulfuric acid (feeding amount is 0.3% of the total mass of citric acid and n-butanol). When the acid value drops below 1 mgKOH/g, it is the end point of esterification. The product is decolorized by activated carbon, filtered, neutralized, washed with water, and distilled under reduced pressure, and the kettle liquid is the finished product.
Refining method: Contains impurities such as free acid and alcohol. During refining, dry with anhydrous potassium carbonate or sodium sulfate and then distill under reduced pressure.
2. Preparation method:
Add 315g (1.0mol) of citric acid (2), 500g (6.7mol) of n-butanol, and 1.5mL of concentrated sulfuric acid into a 1L round-bottomed flask. . Install a 60cm fractionating column and water separator, heat the reaction to dehydrate, and dehydrate 115-120mL in about 4 hours, and the temperature of the reaction solution reaches 130°C. Continue to heat to the reaction solution temperature of 155°C, and recover butanol. Then cool to 50°C, add 4g of solid sodium carbonate in batches to neutralize the sulfuric acid until the pH is 5-6. Leave it overnight, filter, and distill under reduced pressure. Collect the fraction at 198~200℃/0.8~0.9kPa to obtain 460g of product (1) with a yield of 85%. [1]
Purpose
This product is suitable as a plasticizer for polyvinyl chloride, chlorinated alkylene copolymers and cellulose resins. Good compatibility, high plasticizing efficiency; excellent cold resistance, light resistance, and water resistance; low volatility, non-toxic, and mildew resistance. It can also be used as a plastic plasticizer in food packaging, medical and sanitary products, and children’s toys. This product is a plasticizer for polyvinyl chloride and various cellulose resins. Non-toxic. Good compatibility and high plasticizing efficiency. Excellent cold resistance, light resistance and water resistance. Low volatility. Can be used as food packaging materials and medical and sanitary products. Poor electrical insulation properties. Used as a plasticizer for nitrocellulose and cellulose acetate coatings, it can increase the oil resistance and good adhesion of the coating film. It is also used as a defoaming agent and rust inhibitor for protein solutions.

 微信扫一扫打赏
微信扫一扫打赏

