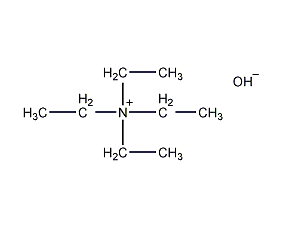
Structural formula
| Business number | 01MK |
|---|---|
| Molecular formula | C8H21NO |
| Molecular weight | 147.26 |
| label |
Tetraethylamine hydroxide, N,N,N-triethylammonium hydroxide, Tetraethylammonium hydroxide solution, Tetraethylammonium hydroxide methanol, TEA Hydroxide, Used as polarographic analysis reagent |
Numbering system
CAS number:77-98-5
MDL number:MFCD00009024
EINECS number:201-073-3
RTECS number:KH3150000
BRN number:3912961
PubChem number:24888410
Physical property data
1. Properties: The product is a 20% aqueous solution, which is a colorless or light yellow liquid.
2. Density (g/mL, 25/4℃): 1.023
3. Relative vapor density (g/mL, air=1): Uncertain
3. p>
4. Melting point (ºC): 40~50°C
5. Boiling point (ºC, normal pressure): 110°C
6. Boiling point (ºC, normal pressure) 5.2kPa): Uncertain
7. Refractive index: 1.4020
8. Flash point (ºC): 11°C
9. Specific optical rotation Degree (º): -44° (c=11.2, H2O)
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: soluble in water.
Toxicological data
1. Acute toxicity
Mouse subcutaneous LC50: 107 mg/kg;
Frog gastrointestinal LDLO: 60 mg/kg;
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 47.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Corrosive.
Storage method
This product should be kept sealed.
Synthesis method
1. First dissolve tetraethylammonium chloride in methanol, then add the theoretical amount of potassium hydroxide-methanol solution, mix thoroughly, and there will be potassium chloride Precipitation:
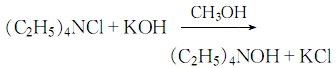
When the reaction is complete, let it stand and filter under reduced pressure. Add water to the resulting filtrate for vacuum distillation to remove alcohol and water, so that the tetraethylammonium hydroxide content in the solution reaches 44% , and then dried under vacuum, the resulting crystal is the finished product.
2. First dissolve tetraethylammonium iodide in water to make it a 15% solution, then add a theoretical amount of silver oxide, heat and keep warm in a water bath to complete the reaction:
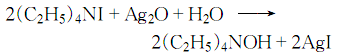
Filter out the silver iodide, and the clean filtrate obtained is the finished product.
Purpose
1. Used as chemical reagents and acetylation of nucleosides, etc.
2.Used as polarographic analysis reagent to support electrolyte.

 微信扫一扫打赏
微信扫一扫打赏

