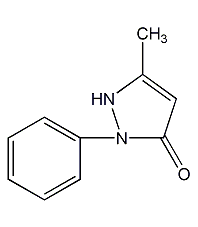
Structural formula
| Business number | 01BL |
|---|---|
| Molecular formula | C11H12N2O |
| Molecular weight | 188.23 |
| label |
2,3-Dimethyl-1-phenyl-3-pyrazolin-5-one, Phenazone, 1,5-Dimethyl-2-phenyl-3-pyrazolinone, antipyraline, Dimethylphenylpyrazolone, Antipyrine, nona sect, 2,3-Dimethyl-1-phenyl-3-pyrazolin-5-one, Analytical identification reagents |
Numbering system
CAS number:60-80-0
MDL number:MFCD00003146
EINECS number:200-486-6
RTECS number:CD2450000
BRN number:157775
PubChem number:24891029
Physical property data
1. Properties: White flaky crystals or powder. Slightly bitter.
2. Density (g/mL, 25/4℃): 1.0747
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 111-113
5. Boiling point (ºC, normal pressure): 319
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: 1.5697
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: 1g product dissolves in no more than 1ml of water, 1.3ml Ethanol, 1ml chloroform, 43ml ether. Aqueous solutions are neutral to litmus.
Toxicological data
Oral LD50 in rats: 1.8g/kg.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 54.55
2. Molar volume (cm3/mol): 162.7
3. Isotonic specific volume (90.2K): 416.1
4. TableSurface tension (dyne/cm): 42.7
5. Polarizability (10-24cm3): 21.62
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 23.6
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 267
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be kept sealed, dry and protected from light.
Synthesis method
It is obtained by methylation and hydrolysis of 1-phenyl-3-methylpyrazolone-5. In a dry methylation tank, add pyrazolone, then add dimethyl sulfate, raise the temperature to 160°C, react for 6 hours, and heat water to boil for 2 hours. Put the methylation reaction product into a hydrolysis tank containing sodium hydroxide solution, and stir and hydrolyze at 100-110°C for 3 hours. Leave to separate and separate, take the antipyrine oil layer and place it in a crystallization tank, add distilled water to dilute, stir, cool to below 10°C, precipitate crystals, filter, wash, and spin dry to obtain crude antipyrine. The crude product is recrystallized with distilled water and decolorized with activated carbon to obtain the finished product. The methylation operation can also use methyl chloride or methyl bromide as raw materials. This methylation reagent is made into a methanol solution, and stirred and reacted with 1-naphthyl-3-methylpyridone in a slight excess. Then evaporate the methanol, dissolve the reactant in water, make it slightly alkaline with sodium hydroxide, extract antipyrine with benzene, recrystallize it from benzene, and finally purify it with activated carbon and recrystallize it with water. Another method is to use equal moles of N, N-phenylmethylhydrazine and ethyl acetoacetate to heat and reflux in an oil bath at 130-160°C. Antipyrine is extracted from a thick oily liquid with boiling water and then evaporated to remove the water to obtain crystals.
Purpose
1. Analytical reagents for nitric acid, nitrous acid and iodine. Determination of elements that can form complex cations (such as bismuth, tin, antimony and mercury, etc.). Gravimetric determination of titanium. This product is an antipyretic and analgesic drug. This type of non-steroidal anti-inflammatory drug has strong antipyretic, analgesic, anti-inflammatory and anti-rheumatic effects. However, due to serious adverse reactions, clinical applications have been gradually reduced, and some of the old varieties have gradually been phased out. Antipyrine, aminopyrine, metamizole and other tablets were all eliminated drugs announced by the Ministry of Health in September 1982. Antipyrine is an analytical reagent. It is used for the analysis of nitric acid, nitrous acid and iodine, for the determination of elements forming complex cations, and for the gravimetric determination of titanium.
2.Used as an analytical reagent for the analysis of nitric acid, nitrous acid and iodine, the determination of elements forming complex cations, and the gravimetric determination of titanium. It is also used as an antipyretic and analgesic, with strong antipyretic, analgesic, anti-inflammatory and anti-rheumatic effects. However, due to serious adverse reactions, clinical applications have been gradually reduced, and some of the old varieties have gradually been phased out. Antipyrine, aminopyrine, metamizole and other tablets were all eliminateddrugs announced by the Ministry of Health in September 1982.

 微信扫一扫打赏
微信扫一扫打赏

