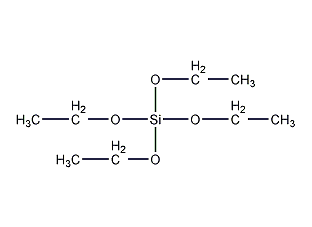
Structural formula
| Business number | 01MR |
|---|---|
| Molecular formula | C8H20O4Si |
| Molecular weight | 208.33 |
| label |
Tetraethyl silicate, Ethyl silicate, Tetraethoxysilane, Tetraethyl silicate, Tetraethyl silicate, Orthosilic acid tetraethyl ester, Tetraethoxy silane, Ethyl orthoxilicate, silicone solvent, weathering-resistant agent, hardener, Precision casting binder |
Numbering system
CAS number:78-10-4
MDL number:MFCD00009062
EINECS number:201-083-8
RTECS number:VV9450000
BRN number:1422225
PubChem number:24888698
Physical property data
1. Properties: colorless transparent liquid with slight odor. [1]
2. Melting point (℃): -77[2]
3. Boiling point (℃): 165~169[3]
4. Relative density (water=1): 0.93[4]
5. Relative vapor density (air=1): 7.22[5]
6. Saturated vapor pressure (kPa): 0.13 (20℃)[6]
7. Octanol/water partition coefficient: 0.04[7]
8. Flash point (℃): 43 (OC); 37.2 ( CC)[8]
9. Ignition temperature (℃): 260[9]
10. Explosion limit (%): 575[10]
11. Lower explosion limit (%): 0.9[11]
12. Solubility: Slightly soluble in water, slightly soluble in benzene, soluble in ether, miscible in ethanol. [12]
13. Viscosity (mPa·s, 20ºC): 17.9
14. Refractive index (20ºC): 1.3928
Toxicological data
1. Acute toxicity[13] LD50: 6270mg/kg (rat oral); 6.3ml (5859mg)/kg (rabbit transdermal)
2. Irritation [14]
Rabbit transdermal: 500mg (24h), severe irritation.
Rabbit eye: 100mg, mild irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 54.80
2. Molar volume (cm3/mol): 221.7
3. Isotonic specific volume (90.2K ): 488.2
4. Surface tension (dyne/cm): 23.4
5. Polarizability (10-24cm3): 21.72 p>
Compute chemical data
1. Number of hydrogen bond donors: 0
2. Number of hydrogen bond acceptors: 4
3. Number of rotatable chemical bonds: 8
4. Topological molecular polar surface area (TPSA): 36.9
5. Number of heavy atoms: 13
6. Surface charge: 0
7. Complexity: 91.2
8. Number of isotope atoms: 0
9. Determine the number of atomic stereocenters: 0
10. The number of uncertain atomic stereocenters: 0
11. The number of determined chemical bond stereocenters: 0
12. Uncertain chemical bond stereocenters Quantity: 0
13. Quantity of covalent bond units: 1
Properties and stability
1. Stability[15] Stable
2. Incompatible substances[16] Strong oxidants, strong acids, strong bases
3. Conditions to avoid contact[17] Water
4. Polymerization hazard[18] No polymerization
5. Decomposition products[19] Silicon oxide
Storage method
Storage Precautions[20] Stored in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. In a 1000mL three-necked flask equipped with a reflux condenser, mechanical stirrer and dropping funnel, add 85g (0.5mol) silicon tetrachloride and 200mL ether, and cool in an ice bath. 92g (2mol) absolute ethanol was added dropwise under stirring. After the addition, heat to reflux on a water bath for 2 hours. After cooling, add sodium ethoxide to neutralize to neutrality, then evaporate the diethyl ether on a water bath, and then perform fractional distillation to obtain 88 g of the product tetraethyl orthosilicate, with a yield of 85%.
2. Preparation method: Silicon tetrachloride and anhydrous ethanol are esterified at normal temperature and pressure, HCL is removed by distillation, and the finished product is obtained after cooling, neutralization, and distillation:
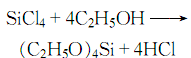
Purpose
1. Used as electrical insulation materials, coatings, and optical glass treatment agents. Also used in organic synthesis.
2. Used in the manufacture of chemical-resistant coatings, heat-resistant coatings, silicone solvents and precision casting adhesives.
3. Used as heat-proof coatings, chemical-resistant coatings, and organic synthesis intermediates. [21]

 微信扫一扫打赏
微信扫一扫打赏

